Respiration kinetic of mango (Mangifera indica L.) as function of storage temperature
Cinética de respiración de mango (Mangifera indica L.) como función de la temperatura de almacenamiento
DOI:
https://doi.org/10.15446/rfna.v69n2.59143Keywords:
Shelf life, Ripeness, Respiratory process (en)Vida útil, Madurez, Proceso respiratorio (es)
DOI: https://doi.org/10.15446/rfna.v69n2.59143
Respiration kinetic of mango (Mangifera indica L.) as function of storage temperature
Cinética de respiración de mango (Mangifera indica L.) como función de la temperatura de almacenamiento
Camilo Agudelo1, Caludia Restrepo2 and José Edgar Zapata1
1 Facultad de Ciencias Farmacéuticas y Alimentarias. Universidad de Antioquia. Calle 67 No. 53-108. Box Air 1226, Medellín, Colombia. <jedgar_4@yahoo.es>
2 Instituto de Ciencia y Tecnología Alimentaria (Foundation INTAL). Carrera 50G No. 12 Sur-91, Itagüí, Colombia.
Received: March 7, 2016; Accepted: July 13, 2016
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

ABSTRACT
Respiration of cut mango (Mangifera Indica L.) cv. Tommy Atkins was studied using the closed system method at three temperatures (4, 20 and 35 °C). Two models were used to estimate the gas concentration, which were adjusted through non-lineal regression algorisms using Matlab R2011a software. Three mathematic models, a model based on Michaelis-Menten's enzymatic kinetics, and two models based on regression analysis, in one of which a saturation equation was included as a new proposal in this field, were set to predict the substrate respiration rate. Results made evident the positive effect of temperature on mango respiration rate. The model with the best adjustment to mango respiration rate was Michaelis-Menten's with an adjusted correlation coefficient of 0.9811 and 0.9747 for CO2 and O2 respectively, with a relative mean error lower than 10%.
Key words: Shelf life, Ripeness, Respiratory process
RESUMEN
Se estudió la respiración del mango cortado (Mangifera Indica L.), cv. Tommy Atkins, utilizando el método de sistema cerrado, a tres temperaturas (4, 20 y 35 °C). Se usaron dos modelos para estimar la concentración de gas, los cuales se ajustaron a través de algoritmos de regresión no lineal usando el software Matlab R2011a. Se ajustaron tres modelos matemáticos para predecir la tasa de respiración del sustrato. Un modelo basado en la cinética enzimática de Michaelis-Menten, y dos modelos basados en análisis de regresión, en uno de los cuales se incluyó una ecuación de saturación como una nueva propuesta en este campo. Los resultados evidenciaron el efecto positivo que tiene la temperatura sobre la tasa de respiración del mango. El modelo que mejor ajuste entregó para la velocidad de respiración del mango fue el modelo de Michaelis-Menten con un coeficiente de correlación ajustado de 0.9811 y 0.9747 para CO2 y O2, respectivamente, con un error medio relativo inferior al 10%.
Palabras claves: Vida útil, Madurez, Proceso respiratorio
Mango (M. indica L.) is one of the most popular subtropical fruits (Souza et al., 2015), because of its high economical value (Sellamuthu et al., 2013), attractive color, delicious taste, rich aroma, exotic flavor, and high nutritional value. Furthermore mango is a rich source of carotenoids and provides high contents of ascorbic acid and phenolic compounds (Shahnawaz et al., 2012; Liu et al., 2013).
In general, fruits and vegetables stay metabolically active after harvest, period during which anabolic processes such as photosynthesis, flavor synthesis, fermentation and other cell wall degraders happen (Heydari et al., 2010). Respiration is of particular interest in postharvest technologies because it is associated to fruit and vegetable quality (Nicolaï et al., 2005). Respiration rate is an indicator of the metabolic level, as high rate respiration is associated with short shelf life (Lurie and Crisosto, 2005), and its control may be an effective mean to regulate the metabolism in general, this is why it is important to extend these products shelf life (Kan et al., 2010). This process changes according to species, variety, storage temperature and vegetable physiological state (Nicolaï et al., 2009). All chemical reactions during ripeness are delayed by low temperatures, these are some of the factors affecting quality and deterioration of fruits and vegetables during storage (Nicolaï et al., 2009) besides the effect on fungi, bacteria and insect growing (Nicolaï et al., 2005).
On the other hand, respiration rate may be reduced by changing the atmosphere around the food, which may also delay associated deterioration reactions, extending fruit and vegetable product shelf life (Luo et al., 2011; Selcuk and Erkan, 2015). For instance, a drop on O2 concentration results in a reduction of the metabolism of broccoli, carrot, pear, tomato (Nicolaï et al., 2005) and papaya (Zapata et al., 2004) among others. CO2 may act as respiration suppressor in vegetables such as apples, broccoli (Nicolaï et al., 2005), mango (Ravindra and Goswami, 2008) and banana (Bhande et al., 2008), while it does not present any inhibitory effect in onions, lettuce and spinach (Nicolaï et al., 2005). Oxygen negative effects occur because reactive oxygen accumulation damages the integrity of mitochondrial membrane, resulting at the end in an irreversible mitochondrial dysfunction, which is believed to be the main cause of ageing in different kinds of organisms among which postharvest fruits are (Qin et al., 2009).
Respiratory process modeling is an important step in designing and selecting packaging and storing systems of fruit and vegetable products as it is the case of modified atmosphere packaging (Ravindra and Goswami, 2008). Then again, works oriented to assess this kind of parameters in fruit and vegetables, and in particular cut products, are rare. Accepting respiratory process modeling with all implicated factors in enzyme reaction would be highly complex and little practical, the usual strategy has been to develop empiric models for each product with controlled variable functions such as temperature or gas concentration (Guevara et al., 2006; Rai and Paul, 2007; Bhande et al., 2008).
The shelf life of minimally processed (cut) products is, then again, particularly important because it is one of the major growing sectors in food retail market (Robles-Sánchez et al., 2013), although the freshly cut mango still has a very limited offer in the world market (Souza et al., 2015). Nevertheless, the popularity of tropical fruits in the most important world markets, is an excellent opportunity for the introduction of fresh-cut mango (Siddiq et al., 2013). Yet, fresh-cut processing induces chemical and biochemical changes as well as increases product respiration rate leading to a reduction of shelf life (Azarakhsh et al., 2014). Therefore, the current work goal is to assess different models to predict respiration rate and gas concentrations of cut mango cv. Tommy Atkins variety at three different temperatures in a closed system.
MATERIALS AND METHODS
Raw material preparation
Mango (Mangifera indica L.) fruits cv. Tommy Atkins in physiological ripe state determined by Colombian technical norm NTC 5210 (ICONTEC, 2003) were used. These were bought in Central Minorista in the city of Medellín, to which they were brought from the southwest of the department of Antioquia. They were washed with alkaline detergent and disinfected with a solution at 100 ppm of sodium hypochlorite (Ngarmsak et al., 2005). They were cut in 0.5 cm edge cubes, leaving seed and epidermis aside, samples were taken to determine pH (ICONTEC, 1991), soluble solids (ICONTEC, 1999), and acidity contents expressed as citric acid percentage (ICONTEC, 1991).
Respiration experimental calculation
Respiration rate was determined to the mango in a closed system (Ravindra and Goswami, 2008), which consisted in subjecting 150 g of the product deposited in a 4,000 mL capacity airtight recipient to atmospheric conditions of 20.94 % O2, 78.08 % N2, and 0.03 % CO2.
Three temperatures were set out in 4, 20 and 35 °C using an oven with temperature control (Binder, Germany). O2 consumption and CO2 production were measured by a PBI Dansensor Check Point O2/CO2 analyzer (Dansensor, Denmark). Each assay was performed twice.
Expressions (1) and (2) were used to calculate respiration rate, these had already been used in products such as pepper (Artés-Hernández et al., 2010), mango (Ravindra and Goswami, 2008), and banana (Bhande et al., 2008).
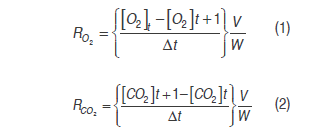
Where RO2 and RCO2 are the respiration rates (mL kg-1 h-1), [O2] and [CO2] are oxygen and carbon dioxide concentrations, t is time (h), Δt is the time between the two measures of the respective gas, V is the free volume in the respiration chamber (mL), and W is the cut fruit mass (kg).
Models for the gas behavior
The following models were used to estimate the gas concentration by a regression made with Matlab R2011a software, finding the model coefficients for all treatments.
Model 1. A model is proposed to estimate the experimental data of the O2 and CO2 concentrations by a growing reason expression or saturation equation (Chapra and Canale, 2007), as indicated in equations (3) and (4), adjusting them to CO2 kinetic production and O2 consumption, respectively.
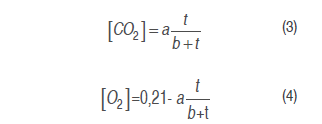
Where a and b are model parameters, and t is time (h).
Model 2. The model used by Bhande et al. (2008) in banana, and Ravindra and Goswami (2008) in mango cv. Amrapali, was also assessed. Equations (5) and (6) are used in this one adjusting O2 and CO2 concentrations in time function.
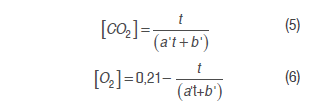
Where a' and b' are model parameters and t is time (h).
Respiration models
Respiration rate for CO2 (RCO2) and O2 (RO2) was calculated by three different methodologies:
Equations (3), (4), (5) and (6) were derived, as derivatives of model 1and model 2, respectively.
Respiration rate was calculated with model 1 and model 2 derivatives, replacing them in equations (7) and (8), as follows:
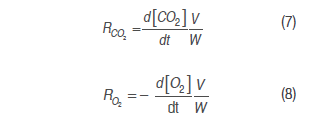
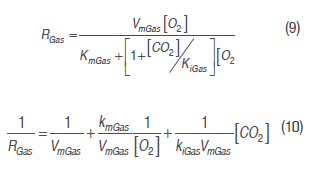
To obtain the cut mango respiration rate in the described conditions, a model based on Michaelis-Menten's kinetic equation was used, using enzymatic kinetic noncompetitive inhibition principles, where CO2 is supposed to react to an enzyme-substrate complex (Artés-Hernández et al., 2010; Ravindra and Goswami, 2008; Bhande et al., 2008). Equation (9) expresses this mechanism for the respiration process in terms of O2 consumption and CO2 production rate. This kind of model presented a significant adjustment in the calculation of mango (Ravindra and Goswami, 2008), banana (Bhande et al., 2008), pepper (Artés-Hernández et al., 2010) and other fruit (Fonseca et al., 2002) respiratory activity.
Arrhenius's equation
Temperature effect on O2 consumption and CO2 production rate was assessed by Arrhenius's equation (Iqbal et al., 2009; Waghmare et al., 2013).
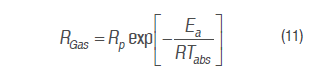
Where RGas is O2 consumption and CO2 production rate (mL kg-1h-1), Rp is respiration pre-exponential factor,Ea is the activation energy (J mol-1), R is the gas universal constant (8,314 J mol-1 K-1), and Tabs is the absolute temperature in K.
Relative standard deviation
Respiration rates predicted from the different models were contrasted with the experimental respiration rate, equations (1) and (2) by relative standard deviation, equation (12).
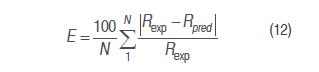
Where E is the relative standard deviation module (%), N is the respiration data point number, Rexp is the experimental respiration rate (mL kg-1 h-1), and Rpred is the predicted respiration rate (mL kg-1 h-1). A good adjustment is defined with E<10 % (Ravindra and Goswami, 2008).
RESULTS AND DISCUSION
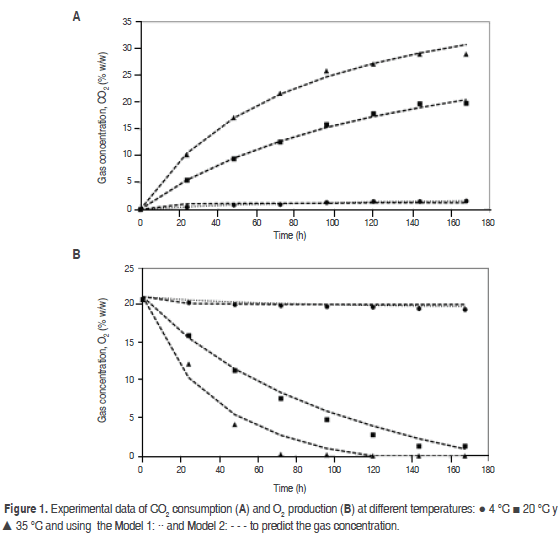
The physicochemical parameters to fresh mango were pH 3.4, °Bx 9.5 and acidity 1.05% as citric acid. Experimental data of the O2 consumption and CO2 production at different temperatures are shown in figure 1. The nonlinear drop on O2 concentration in the conditions of the current work matches the pattern expected from other climacteric products kept in sealed containers (Ravindra and Goswami, 2008; Hagger et al., 1992; Jacxsens et al., 1999; Guevara et al., 2006) and agrees with the hypothesis of saturation equation (model 1), as this type of equations start assuming that the system is saturated with CO2 and O2 consumed until exhaustion (Chapra and Canale, 2007). It is observed that a rise in temperature also raises the gradient of the graph for the gas behavior, consequently the respiration rate increases as it happens with the papaya (Zapata et al., 2004), banana (Bhande et al., 2008), and mango cv. Amrapali (Ravindra and Goswami, 2008). This is due to that the rate of the enzymatic reactions grows exponentially with the rise of the temperature (Lee et al., 1996). Low temperatures reduce respiration rate, O2 consumption, CO2 and ethylene production. As the tissues react to the latter (Mendoza et al., 2016), in order for maturity to occur, the needs of ethylene and the time of exposure are higher at low temperatures (Kader, 1994).
Models 1 and 2 parameters
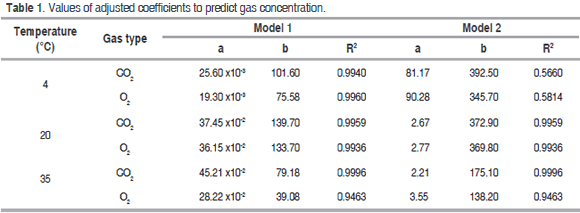
Table 1 shows the values of the coefficients a and b for equations (3), (4), (5) and (6) with their respective correlation coefficients (R2), obtained for the three temperatures assessed in the closed system. Model 1 presented an excellent adjustment to the data in the three assessed temperatures, while model 2 had a very poor adjustment to the data at 4 °C. Model 2 coefficients presented a high variability with temperature compared with model 1; it can be seen that parameter b is more influenced with temperature than coefficient a in both.
Respiration kinetics
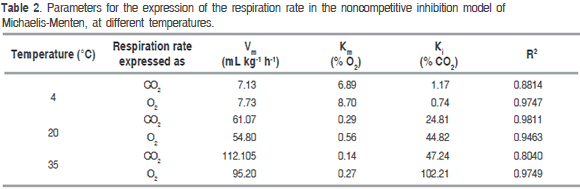
Table 2 presents Michaelis-Menten's model parameters, with their corresponding adjusted correlation coefficient (R2). A good adjustment of the experimental data with such model for RCO2 and RO2 is observed.
Table 2 data analysis based on the form of equation (9), allows to observe the effect gases have on the rate of CO2 production and O2 consumption. These effects are more noticeable at low CO2 and high O2 concentrations, at 20 and 35 °C. Km and Ki values indicate CO2 production rate is more sensible to the concentration of both gases than O2 consumption rate, due to both constants are lower for the CO2 production than O2 consumption expressions. On the other hand, low Km values indicate the significant effect of O2 concentration on respiration rates in terms of CO2 as well as O2. While relatively high Ki values indicate that CO2 concentration does not have such as significant effect on respiration rates. Nevertheless, the effect this gas has on respiration rate, given the adjust the model shows, cannot be denied. Besides, it is known that very high CO2 and very low O2 concentrations may cause respiration to change from aerobic to anaerobic, causing the creation of fermentation products such as acetaldehyde and ethanol (Angós et al., 2008).
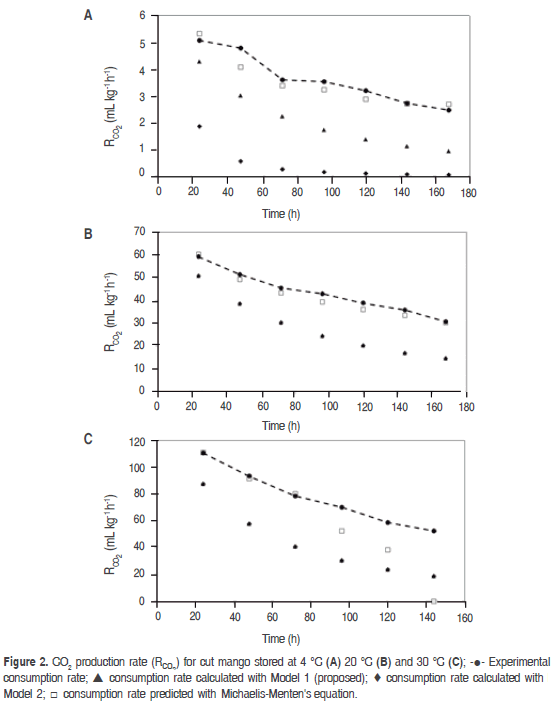
Figure 2 shows that CO2 experimental consumption rate (RCO2) has a starting value at 4 °C of 5.09 mL kg-1 h-1, compared to the same gas experimental rate at 20 °C of 59.21 mL kg-1 h-1, and at 35 °C it is of 110.76 mL kg-1 h-1. This difference is due to reduction of temperature becomes into a reduction of the rate at which parameter such as respiration, texture and microbial growth change; this is why the organoleptic and physiological characteristics of mango stored at 4 °C did not present significant change in odor, texture and flavor.
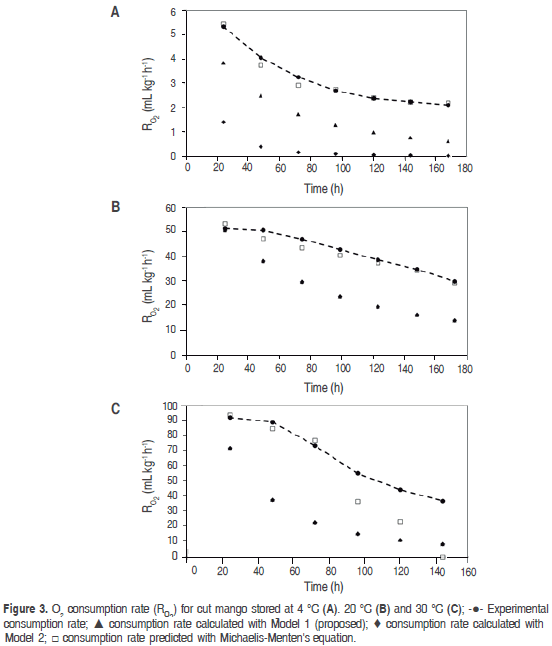
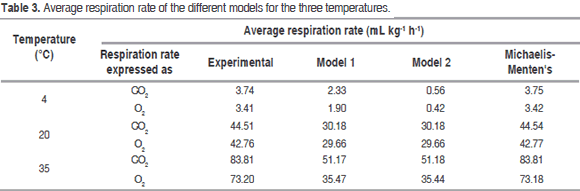
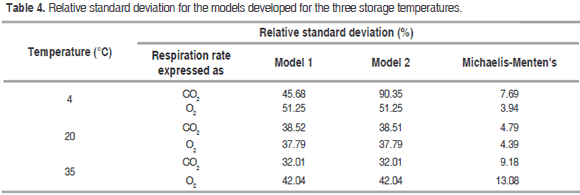
Reduction of temperature not only reduces ethylene production, but also the response rate of tissues to such gas; therefore, the more the temperature drops, the higher exposure time required by the metabolic cycle to start at a certain ethylene concentration is (Mendoza et al., 2016). Associated to this the temperature affect RO2, as can be seen in figure 3.
Table 3 shows the RCO2 and RO2 average value predicted for the three temperatures with Michaelis-Menten's equation, models 1 and 2, being noncompetitive inhibition Michaelis-Menten's equation the best model. Figures 2 and 3 show graphic behavior of experimental respiration and the one predicted by the models in terms of RCO2 and RO2.
The model proposed (Model 1) is the second that best adjusts to O2 consumption and CO2 production, and also to the prediction of RCO2 and RO2, being relevant at 4 °C (Figure 2 (A) for CO2 and Figure 3 (A) for O2); while at 20 °C and 35 °C the proposed model and the one in the literature present the same values predicted of RCO2 and RO2. A cell change due to the incapacity of the enzymes associated to mitochondrial membranes to metabolize glycolysis products may happen at these low temperatures (Kader, 1994) therefore, model 2 may not give a good adjustment.
Relative standard deviation values of each one of the models are shown in table 4. Michaelis-Menten's kinetic model obtained an excellent adjustment for the experimental value with E<10% in the different temperatures, while the model reported in literature had the least fitting, except for RCO2 and RO2 in refrigeration temperature; this is due to this model adjustment expressed in the R2 in table 1 is not adequate for this condition. Model 2, reported by Bhande et al. (2008) and Ravindra and Goswami (2008), could be useful in temperatures higher to 4 °C, but does not present good adjustment.
Table 5 shows Arrhenius type equation parameters, activation energy and pre-exponential factor for O2 consumption and CO2 production rates of the experimental data, proposed model 1 and Michaelis-Menten's equation. Activation energy values of each gas are very alike between experimental data and Michaelis-Menten's, evidencing a good adjustment of this equation to the sensitivity of the reaction to temperature; besides, activation energy values are between normal values, from 29 to 93 kJ mol-1 for fruit (Benítez et al., 2012). Certain effects could be observed in some cut mango pieces due to cold sensitive reactions generating a brown color, beginning around the vascular bundles, because of the polyphenol-oxidase action on the phenolics released out of the vacuole after freezing (Lee et al., 1996; Blanco-Díaz et al., 2016).
The descending form of the gradient in figures 2 and 3 indicates an inverse relationship between respiration rate and shelf life, which is due to O2 reduces and CO2 increases in an airtight container, consequently with what is observed in figure 1. It is also known that respiration rate decreases with CO2 rising and O2 dropping due to the first is a product of the reaction and the second is a reactive in it, as can be seen in the general reaction for respiration (Devanesan et al., 2011):

Consequently and accordingly to Le Chatelier's principle, when increasing the concentration of a product or reducing the concentration of a reactive, the chemical reaction rate reduces.
CONCLUSIONS
Noncompetitive inhibition Michaelis-Menten's kinetic equation presented better results in the prediction of the respiration rate in contrast with others two models.
Models 1 and 2 are useful to predict gas concentration but do not predict the respiration rates.
Temperature was an influencing factor in O2 consumption and CO2 production of the cut mango determining a better preservation at 4 °C.
AKNOWLEDGMENTS
The authors of the current work thank the Comité para el Desarrollo de la Investigación (CODI) in the Universidad de Antioquia, for the financial support through the Estrategia de Sostenibilidad 2014-2015. Also to the Fundación INTAL for lending its facilities.
REFERENCES
Angós I, Vírseda P and Fernández T. 2008. Control of respiration and color modification on minimally processed potatoes by means of low and high O2/CO2 atmospheres. Postharvest Biology and Technology 48(3): 422-430. doi: 10.1016/j.postharvbio.2007.10.019
Azarakhsh N, Osman A, Ghazali H M, Tan C P and Adzahan N M. 2014. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of freshcut pineapple. Postharvest Biology and Technology 88: 1-7.
Artés-Hernández F, Conesa A, and Artés F. 2010. Minimally fresh processed pepper under different kind of cuts. Acta Horticulturae 857: 25-30. doi: 10.17660/ActaHortic.2010.857.1
Benítez S, Chiumenti M, Sepulcre F, Achaerandio L and Pujolá M. 2012. Modeling the effect of storage temperature on the respiration rate and texture of fresh cut pineaple. Journal of Food Engineering 113(4): 527-33. doi: 10.1016/j.jfoodeng.2012.07.022
Bhande SD, Ravindra MR and Goswami TK. 2008. Respiration rate of banana fruit under aerobic conditions at different storage temperature. Journal of Food Engineering 87(1): 116-123. doi: 10. 1016/j.jfoodeng.2007.11.019
Blanco-Díaz MT, Pérez-Vicente A and Font R. 2016. Quality of fresh cut zucchini as affected by cultivar, Maturity at processing and packaging. Packaging Technology and Science 29(7): 365-382. doi: 10.1002/pts.2214
Chapra S and Canale R. 2007. Métodos numéricos para ingenieros. Quinta edición. México. McGraw Hill Interamericana. 977 p.
Devanesan JN, Karuppiah A and Abirami CVK. 2011. Effect of storage temperatures, O2 concentrations and variety on respiration of mangoes. Journal of Agrobiology 28(2): 119-128. doi: 10.2478/v10146-011-0013-8
Fonseca SC, Oliveira FAR and Brecht JK. 2002. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: a review. Journal of Food Engineering 52(2): 99-119. doi: 10.1016/S0260-8774(01)00106-6
Guevara JC, Yahia EM, Beaudry RM and Cedeño L. 2006. Modeling the influence of temperature and relative humidity on respiration rate of prickly pear cactus cladodes. Postharvest Biology and Technology 41(3): 260-265. doi: 10.1016/j.postharvbio. 2006.04.012
Hagger PE, Lee DS and Yam KL. 1992. Application of an enzyme kinetics based respiration model to closed system experiments for fresh produce. Journal of Food Process Engineering 15(2): 143-157. doi: 10.1111/j.1745-4530.1992.tb00148.x
Heydari A, Shayesteh K, Eghbalifam N and Bordbar H. 2010. Studies on the respiration rate of banana fruit based on enzyme kinetics. International Journal of Agriculture and Biology 12(1): 145-149.
ICONTEC. 2003. Norma Técnica Colombiana 5210 Frutas frescas. Mango. Variedades mejoradas. Especificaciones, Instituto Colombiano de Normas Técnicas y Certificación, Bogotá, Colombia.
ICONTEC. 1999. Norma Técnica Colombiana 4624 Jugos de frutas y hortalizas. Determinación del contenido de sólidos solubles. Método refractométrico, Instituto Colombiano de Normas Técnicas y Certificación, Bogotá, Colombia.
ICONTEC. 1991 Norma Técnica Colombiana 440 Productos alimenticios. Métodos de ensayo, Instituto Colombiano de Normas Técnicas y Certificación, Bogotá, Colombia.
Iqbal T, Rodrigues F, Mahajan P and Kerry J. 2009. Mathematical modelling of the influence of temperature and gas composition on the respiration rate of shredded carrots. Journal of Food Engineering 91: 325-332.
Jacxsens L, Devlieghere F and Debevere J. 1999. Validation of a systematic approach to design equilibrium modified atmosphere packages for fresh-cut produce. LWT - Food Science and Technology 32(7): 425-432. doi: 10.1006/fstl.1999.0558
Kader A. 1994. Modified and Controlled Atmosphere Storage of Tropical Fruits. ACIAR. 50: 239-49
Kan J, Wang H, Jin C and Xie H. 2010. Changes of reactive oxygen species and related enzymes in mitochondria respiratory metabolism during the ripening of peach fruit. Agricultural Sciences in China. 9(1): 138-146. doi: 10.1016/S1671-2927(09)60077-8
Lee D, Song Y and Yam K. 1996. Application of an enzyme kinetics based respiration model to permeable system experiment of fresh produce. Journal of Food Engineering 27(3): 297-310. doi: 10.1016/0260-8774(95)00012-7
Liu FX, Fu SF, Bi XF, Chen F, Liao XJ, Hu XS and Wu JH. 2013. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chemistry 138: 396-405. doi: 10.1016/j.foodchem.2012.09.111
Luo Z, Chen C and Xie J. 2011. Effect of salicylic acid treatment on alleviating postharvest chilling injury of 'Qingnai' plum fruit. Postharvest Biology and Technology 62(2): 115-120. doi: 10.1016/j.postharvbio.2011.05.012
Lurie S and Crisosto CH. 2005. Chilling injury in peach and nectarine. Postharvest Biology and Technology 37: 195-208. doi: 10.1016/j.postharvbio.2005.04.012
Mendoza R, Castellanos DA, García JC, Vargas JC and Herrera AO. 2016. Ethylene production, respiration and gas exchange modelling in modified atmosphere packaging for banana fruits. International Journal of Food Science & Technology 51(3): 777-788.
Ngarmsak M, Ngarmsak T, Ooraikul B, Delaquis PJ, Toivonen PM and Mazza G. 2005. Effect of sanitation treatments with heated, chlorinated water on the microbiology of fresh-cut Thai mangoes. ISHS Acta Horticulturae. 682: 1895-1899. doi: 10.17660/ActaHortic.2005.682.255
Nicolaï B, Lammertyn J, Schotsmans W and Verlinden B. 2005. Gas exchange properties of fruits and vegetables. 3 ed. EU. Engineering Properties of Foods CRC. 739-770 pp.
Nicolaï B, Hertog M, Ho Q and Verlinden B. 2009. Gas exchange modeling. 93-108. In: Yahia E, Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities. CRC, New York. 608 p.
Qin G, Wang Q, Liu J, Li B and Tian S. 2009. Proteomic analysis of changes in mitochondrial protein expression during fruit senescence. Proteomics 9(17): 4241-4253. doi: 10.1002/pmic.200900133.
Rai D and Paul S. 2007. Transient state in-pack respiration rates of mushroom under modified atmosphere packaging based on enzyme kinetics. Biosystems Engineering. 98(3): 319-326. doi: 10.1016/j.biosystemseng.2007.07.012
Ravindra MR and Goswami TK. 2008. Modelling the respiration rate of green mature mango under aerobic conditions. Biosystems Engineering. 99(2): 239-48. doi: 10.1016/j.biosystem seng.2007.10.005
Robles-Sánchez RM, Rojas-Graü MA, Odriozola-Serrano I, González-Aguilar G and Martin-Belloso O. 2013. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT - Food Science and Technology 50: 240-246.
Salinas-Hernández RM, González-Aguilar GA and Tiznado-Hernández ME. 2015. Utilization of physicochemical variables developed from changes in sensory attributes and consumer acceptability to predict the shelf life of fresh-cut mango fruit. Journal of Food Science and Technology 52(1): 63-77.
Selcuk N and Erkan M. 2015. The effects of modified and palliflex controlled atmosphere storage on postharvest quality and composition of 'Istanbul' medlar fruit. Postharvest Biology and Technology. 99: 9-19. doi: 10.1016/j.postharvbio.2014.07.004
Sellamuthu PS, Denoya GI, Sivakumar D, Polenta GA and Soundy P. 2013. Comparison of the contents of bioactive compounds and quality parameters in selected mango cultivars. Journal of Food Quality 36: 394-402. doi: 10.1111/jfq.12058
Shahnawaz M, Sheikh SA, Panwr AA, Khaskheli SG and Awan FA. 2012. Effect of hot water treatment on the chemical, sensorial properties and ripening quality of Chaunsa mango (Mangifera indica L.). Journal of Basic Applied Sciences 8: 328-333. doi: 10.6000/1927-5129.2012.08.02.13
Siddiq M, Sogi DS and Dolan KD. 2013. Antioxidant properties, total phenolics, and quality of fresh-cut 'Tommy Atkins' mangoes as affected by different pre-treatments. LWT - Food Science and Technology 53(1): 156-162. doi: 10.1016/j.lwt.2013.01.017
Souza PM, Antônio FM, Cerqueira MA, Teixeira JA, Vicente AA, Carneiro-da-Cunha and Maria G. 2015. Effect of an edible nanomultilayer coating by electrostatic self-assembly on the shelf life of fresh-cut mangoes. Food and Bioprocess Technology 8(3): 647-654. doi: 10.1007/s11947-014-1436-1
Waghmare RB, Mahajan PV and Annapure US. 2013. Modelling the effect of time and temperature on respiration rate of selected fresh-cut produce. Postharvest Biology and Technology 80(0): 25-30.
Zapata J, Carvajal L and Ospina N. 2004. Aplicación de métodos combinados para la conservación de papaya hawiana (Carica papaya) cortada en láminas. Alimentación Equipos y Tecnología. 190: 113-9.
References
Angós I, Vírseda P and Fernández T. 2008. Control of respiration and color modification on minimally processed potatoes by means of low and high O2/CO2 atmospheres. Postharvest Biology and Technology 48(3): 422-430. doi: 10.1016/j.postharvbio.2007.10.019
Azarakhsh N, Osman A, Ghazali H M, Tan C P and Adzahan N M. 2014. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of freshcut pineapple. Postharvest Biology and Technology 88: 1-7.
Artés-Hernández F, Conesa A, and Artés F. 2010. Minimally fresh processed pepper under different kind of cuts. Acta Horticulturae 857: 25-30. doi: 10.17660/ActaHortic.2010.857.1
Benítez S, Chiumenti M, Sepulcre F, Achaerandio L and Pujolá M. 2012. Modeling the effect of storage temperature on the respiration rate and texture of fresh cut pineaple. Journal of Food Engineering 113(4): 527-33. doi: 10.1016/j.jfoodeng.2012.07.022
Bhande SD, Ravindra MR and Goswami TK. 2008. Respiration rate of banana fruit under aerobic conditions at different storage temperature. Journal of Food Engineering 87(1): 116-123. doi: 10. 1016/j.jfoodeng.2007.11.019
Blanco-Díaz MT, Pérez-Vicente A and Font R. 2016. Quality of fresh cut zucchini as affected by cultivar, Maturity at processing and packaging. Packaging Technology and Science 29(7): 365-382. doi: 10.1002/pts.2214
Chapra S and Canale R. 2007. Métodos numéricos para ingenieros. Quinta edición. México. McGraw Hill Interamericana. 977 p.
Devanesan JN, Karuppiah A and Abirami CVK. 2011. Effect of storage temperatures, O2 concentrations and variety on respiration of mangoes. Journal of Agrobiology 28(2): 119-128. doi: 10.2478/v10146-011-0013-8
Fonseca SC, Oliveira FAR and Brecht JK. 2002. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: a review. Journal of Food Engineering 52(2): 99-119. doi: 10.1016/S0260-8774(01)00106-6
Guevara JC, Yahia EM, Beaudry RM and Cedeño L. 2006. Modeling the influence of temperature and relative humidity on respiration rate of prickly pear cactus cladodes. Postharvest Biology and Technology 41(3): 260-265. doi: 10.1016/j.postharvbio. 2006.04.012
Hagger PE, Lee DS and Yam KL. 1992. Application of an enzyme kinetics based respiration model to closed system experiments for fresh produce. Journal of Food Process Engineering 15(2): 143-157. doi: 10.1111/j.1745-4530.1992.tb00148.x
Heydari A, Shayesteh K, Eghbalifam N and Bordbar H. 2010. Studies on the respiration rate of banana fruit based on enzyme kinetics. International Journal of Agriculture and Biology 12(1): 145-149.
ICONTEC. 2003. Norma Técnica Colombiana 5210 Frutas frescas. Mango. Variedades mejoradas. Especificaciones, Instituto Colombiano de Normas Técnicas y Certificación, Bogotá, Colombia.
ICONTEC. 1999. Norma Técnica Colombiana 4624 Jugos de frutas y hortalizas. Determinación del contenido de sólidos solubles. Método refractométrico, Instituto Colombiano de Normas Técnicas y Certificación, Bogotá, Colombia.
ICONTEC. 1991 Norma Técnica Colombiana 440 Productos alimenticios. Métodos de ensayo, Instituto Colombiano de Normas Técnicas y Certificación, Bogotá, Colombia.
Iqbal T, Rodrigues F, Mahajan P and Kerry J. 2009. Mathematical modelling of the influence of temperature and gas composition on the respiration rate of shredded carrots. Journal of Food Engineering 91: 325-332.
Jacxsens L, Devlieghere F and Debevere J. 1999. Validation of a systematic approach to design equilibrium modified atmosphere packages for fresh-cut produce. LWT - Food Science and Technology 32(7): 425-432. doi: 10.1006/fstl.1999.0558
Kader A. 1994. Modified and Controlled Atmosphere Storage of Tropical Fruits. ACIAR. 50: 239-49
Kan J, Wang H, Jin C and Xie H. 2010. Changes of reactive oxygen species and related enzymes in mitochondria respiratory metabolism during the ripening of peach fruit. Agricultural Sciences in China. 9(1): 138-146. doi: 10.1016/S1671-2927(09)60077-8
Lee D, Song Y and Yam K. 1996. Application of an enzyme kinetics based respiration model to permeable system experiment of fresh produce. Journal of Food Engineering 27(3): 297-310. doi: 10.1016/0260-8774(95)00012-7
Liu FX, Fu SF, Bi XF, Chen F, Liao XJ, Hu XS and Wu JH. 2013. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chemistry 138: 396-405. doi: 10.1016/j.foodchem.2012.09.111
Luo Z, Chen C and Xie J. 2011. Effect of salicylic acid treatment on alleviating postharvest chilling injury of 'Qingnai' plum fruit. Postharvest Biology and Technology 62(2): 115-120. doi: 10.1016/j.postharvbio.2011.05.012
Lurie S and Crisosto CH. 2005. Chilling injury in peach and nectarine. Postharvest Biology and Technology 37: 195-208. doi: 10.1016/j.postharvbio.2005.04.012
Mendoza R, Castellanos DA, García JC, Vargas JC and Herrera AO. 2016. Ethylene production, respiration and gas exchange modelling in modified atmosphere packaging for banana fruits. International Journal of Food Science & Technology 51(3): 777-788.
Ngarmsak M, Ngarmsak T, Ooraikul B, Delaquis PJ, Toivonen PM and Mazza G. 2005. Effect of sanitation treatments with heated, chlorinated water on the microbiology of fresh-cut Thai mangoes. ISHS Acta Horticulturae. 682: 1895-1899. doi: 10.17660/ActaHortic.2005.682.255
Nicolaï B, Lammertyn J, Schotsmans W and Verlinden B. 2005. Gas exchange properties of fruits and vegetables. 3 ed. EU. Engineering Properties of Foods CRC. 739-770 pp.
Nicolaï B, Hertog M, Ho Q and Verlinden B. 2009. Gas exchange modeling. 93-108. In: Yahia E, Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities. CRC, New York. 608 p.
Qin G, Wang Q, Liu J, Li B and Tian S. 2009. Proteomic analysis of changes in mitochondrial protein expression during fruit senescence. Proteomics 9(17): 4241-4253. doi: 10.1002/pmic.200900133.
Rai D and Paul S. 2007. Transient state in-pack respiration rates of mushroom under modified atmosphere packaging based on enzyme kinetics. Biosystems Engineering. 98(3): 319-326. doi: 10.1016/j.biosystemseng.2007.07.012
Ravindra MR and Goswami TK. 2008. Modelling the respiration rate of green mature mango under aerobic conditions. Biosystems Engineering. 99(2): 239-48. doi: 10.1016/j.biosystem seng.2007.10.005
Robles-Sánchez RM, Rojas-Graü MA, Odriozola-Serrano I, González-Aguilar G and Martin-Belloso O. 2013. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT - Food Science and Technology 50: 240-246.
Salinas-Hernández RM, González-Aguilar GA and Tiznado-Hernández ME. 2015. Utilization of physicochemical variables developed from changes in sensory attributes and consumer acceptability to predict the shelf life of fresh-cut mango fruit. Journal of Food Science and Technology 52(1): 63-77.
Selcuk N and Erkan M. 2015. The effects of modified and palliflex controlled atmosphere storage on postharvest quality and composition of 'Istanbul' medlar fruit. Postharvest Biology and Technology. 99: 9-19. doi: 10.1016/j.postharvbio.2014.07.004
Sellamuthu PS, Denoya GI, Sivakumar D, Polenta GA and Soundy P. 2013. Comparison of the contents of bioactive compounds and quality parameters in selected mango cultivars. Journal of Food Quality 36: 394-402. doi: 10.1111/jfq.12058
Shahnawaz M, Sheikh SA, Panwr AA, Khaskheli SG and Awan FA. 2012. Effect of hot water treatment on the chemical, sensorial properties and ripening quality of Chaunsa mango (Mangifera indica L.). Journal of Basic Applied Sciences 8: 328-333. doi: 10.6000/1927-5129.2012.08.02.13
Siddiq M, Sogi DS and Dolan KD. 2013. Antioxidant properties, total phenolics, and quality of fresh-cut 'Tommy Atkins' mangoes as affected by different pre-treatments. LWT - Food Science and Technology 53(1): 156-162. doi: 10.1016/j.lwt.2013.01.017
Souza PM, Antônio FM, Cerqueira MA, Teixeira JA, Vicente AA, Carneiro-da-Cunha and Maria G. 2015. Effect of an edible nanomultilayer coating by electrostatic self-assembly on the shelf life of fresh-cut mangoes. Food and Bioprocess Technology 8(3): 647-654. doi: 10.1007/s11947-014-1436-1
Waghmare RB, Mahajan PV and Annapure US. 2013. Modelling the effect of time and temperature on respiration rate of selected fresh-cut produce. Postharvest Biology and Technology 80(0): 25-30.
Zapata J, Carvajal L and Ospina N. 2004. Aplicación de métodos combinados para la conservación de papaya hawiana (Carica papaya) cortada en láminas. Alimentación Equipos y Tecnología. 190: 113-9.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. A. Allegra, G. Sortino, P. Inglese, L. Settanni, A. Todaro, A. Gallotta. (2017). The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig ( Ficus carica L.) fruit. Food Packaging and Shelf Life, 12, p.135. https://doi.org/10.1016/j.fpsl.2017.04.010.
2. José Edgar Zapata Montoya, Camilo Agudelo Cuartas, Claudia Restrepo. (2018). Modelamiento de la respiración del mango (Mangifera indica l.) usando el método de sistema cerrado a diferentes temperaturas. Revista Brasileira de Fruticultura, 40(3) https://doi.org/10.1590/0100-29452018126.
3. Johanna Garavito, Aníbal O. Herrera, Diego A. Castellanos. (2021). A combined mathematical model to represent transpiration, respiration, and water activity changes in fresh cape gooseberry (Physalis peruviana) fruits. Biosystems Engineering, 208, p.152. https://doi.org/10.1016/j.biosystemseng.2021.05.015.
4. Manju Joseph, Hui Xiao, Annelies Postelmans, Maarten Hertog, Pieter Verboven, Bart Nicolaï, Wouter Saeys. (2024). Efficient estimation of gas exchange and respiration kinetics in apple using pathlength-resolved GASMAS. Postharvest Biology and Technology, 213, p.112903. https://doi.org/10.1016/j.postharvbio.2024.112903.
5. Dolores Rovira, Claudia Alfaro, Violeta Martínez, Isela Menjívar. (2019). Respiration rate and shelf-life study of Crotalaria longirostrata (chipilín). Journal of Food Measurement and Characterization, 13(4), p.3025. https://doi.org/10.1007/s11694-019-00224-2.
6. Guillermo M. Badillo, Luis A. Segura-Ponce. (2020). Classic and Reaction-Diffusion Models Used in Modified Atmosphere Packaging (MAP) of Fruit and Vegetables. Food Engineering Reviews, 12(2), p.209. https://doi.org/10.1007/s12393-020-09214-3.
7. D Rahayu, N Bintoro, A D Saputro. (2021). Modelling the respiration rate of mango (cv. Manalagi) during storage under various temperatures and gas compositions. IOP Conference Series: Earth and Environmental Science, 653(1), p.012018. https://doi.org/10.1088/1755-1315/653/1/012018.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2016 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.