Study of the crystallization and polymorphic structures formed in oleogels from avocado oil
Estudio de la cristalización y de las estructuras polimórficas formadas en oleogeles de aceite de aguacate
DOI:
https://doi.org/10.15446/rfna.v69n2.59139Keywords:
Calorimetry, Monodiglycerides, Cooling rate, Thermal behavior (en)Calorimetría, Monodiglicérido, Rapidez de enfriamiento, Comportamiento térmico (es)
DOI: https://doi.org/10.15446/rfna.v69n2.59139
Study of the crystallization and polymorphic structures formed in oleogels from avocado oil
Estudio de la cristalización y de las estructuras polimórficas formadas en oleogeles de aceite de aguacate
Ezequiel J. Pérez-Monterroza1, Héctor J. Ciro-Velásquez1 and Julio César Arango Tobón1
1 Facultad de Ciencias Agrarias. Universidad Nacional de Colombia. A.A. 1779, Medellín, Colombia. <eperez494@gmail.com>
Received: February 10, 2016; Accepted: June 30, 2016
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

ABSTRACT
The effect of the concentration of monodiglycerides, beeswax and sorbitan monostearate (SMS) on the thermal behavior of oleogels prepared from avocado oil, as well as the effect of the cooling rate on the onset temperature (Tonset) and the crystallization temperature (Tc), were evaluated by DSC and X-ray diffraction. The results showed that the structuring agents have a significant effect (P<0.05) on the Tc and Tonset, which demonstrated their ability to form solid lipids. Moreover, it was found that the presence of SMS decreased the Tc and Tonset, possibly due to their cocrystallization process. It was found only in the presence of the polymorphic form b' in the oleogels prepared. The Tc varied between 1.6 and 51.4 °C and Tonset between 3.9 and 53.8 °C.
Key words: Calorimetry, Monodiglycerides, Cooling rate, Thermal behavior
RESUMEN
Se evaluó el efecto de la concentración de los monodiglicéridos, la cera de abejas y monoestearato de sorbitán (SMS) sobre el comportamiento térmico de los oleogeles de aceite de aguacate, así como el efecto de la velocidad de enfriamiento sobre el inicio (Tonset) y temperatura de cristalización (Tc), usando DSC y difracción por rayos x. Los agentes estructurantes mostraron tener efecto significativo (P<0,05) sobre la Tonset y la Tc del aceite de aguacate, lo que indica su capacidad de formar estructura. La presencia de Span 60 disminuyó la Tonset y la Tc, posiblemente debido su proceso de cocristalización. Se evidenció únicamente la presencia de la forma polimórfica b' en los oleogeles preparados. La Tc y la Tonset varió en el rango entre 1,6-51,4 °C y 3,9-53,8 °C respectivamente.
Palabras claves: Calorimetría, Monodiglicérido, Rapidez de enfriamiento, Comportamiento térmico
In the industry sector there is a clear trend towards the development of new and healthier food products. New ways for reducing the risk of diseases related to trans fats, has been motivation for the development of solid lipids from edible oils containing structuring agents, but that do not contain trans fats (Da Pieve et al., 2011). These solid lipids are produced from commercial grade additives and are called oleogels. The Oleogels are defined as an organic liquid confined in a thermoreversible three-dimensional network created by an organogelator. Typically, these structures are formed from very low concentrations of organogelator and their ability to form structures is dependent on the nature and symmetry of the molecule, its molecular weight, and the difference in solubility to the solvent (Omonov et al., 2010; Raut et al., 2011; Pernetti et al., 2007; Dassanayake et al., 2011). In the food industry oleogels have been produced from a variety of compounds, such as vegetable waxes, monodiglycerides, alcohols or esters of fatty acids, phospholipids and sterols. There is a close relationship between the structure of the organogelator agent and the composition of the edible oils used, which influences the microstructural and physical chemical characteristics of the oleogel (Toro-Vazquez et al., 2010; Murdan et al., 1999b; Bot et al., 2011). The chain fatty acids in triacylglycerols (TAGs) are forced into alignment, grouping and leading to the formation of several polymorphic structures. Vegetable fats and oils contain basically three polymorphic forms: α, b', and b, the first is the least stable structure, and the thermodynamically more stable is the b-form (Marangoni, 2002; Sato and Ueno, 2011; Himawan et al., 2006; Ortiz-Moreno et al., 2003). The aim of the present study was to evaluate by DSC technique and X-ray diffraction the effect of the concentration of the organogelators such as monoglycerides, beeswax, sorbitan monostearate and cooling rate on the onset temperature (Tonset) and crystallization temperature (Tc) in the avocado oil oleogels.
MATERIALS AND METHODS
Extraction of avocado oil
This process was carried out using the methodology proposed by Betancur (2011) with some modifications. The ripe avocado (Persea americana Mill. cv. Hass) was purchased from a local market; it was washed, peeled and pulped. The pulp was liquefied with water to prepare a solution of avocado to 15%, the oil was then extracted using a centrifuge (Hettich, Universal 320 R) at 3500 rpm for 15 min, thereby separating the lipid fraction from the soluble fraction, unlike Betancur who used a cream separator at 8070 rpm at 50 °C. The extracted oil was stored at 5 °C and subsequently used to prepare oleogels.
Preparation of oleogels
The methodology of oleogel preparation was developed based on previous works (Lupi et al., 2012; Morales-Rueda et al., 2009) with modifications. The oleogels (100 g) were prepared with different concentrations of organogelators (commercial grade): monoglyceride (monoester 99%), beeswax and sorbitan monostearate (SMS). Avocado oil was preheated to 75 °C on a hot plate. The amounts of organogelator, according to the experimental design given in table 1, were weighed. They were then mixed with the avocado oil for 10 min to ensure that the solid components reached their melting points and that the proper homogenization of the blend was achieved. The oleogels were allowed to stand at a temperature of 5 °C for 24 h to achieve the thermal characterization. Two replicate experiments were conducted for each test.
Thermal characterization: Differential Scanning Calorimetry (DSC)
The thermal characterization of the oleogels was conducted by analyzing the thermogram obtained in a DSC TA Q2000 (TA Instruments, New Castle, Delaware). The equipment was calibrated with indium before analysis. Nitrogen was used as a purge gas for the system 5 × 10-5 m3 min-1. For the analysis, 12-15 mg of oleogel was weighed in aluminum pans. An empty aluminium pan was used as reference. To eliminate any memory structure, the samples were heated and kept at 75 °C for 10 min, then cooled at 3 or 5 °C min-1 to -20 °C (Table 1). The results of thermal analysis were processed using TA Universal Analysis 2000 software (TA Instruments, New Castle, Delaware). Each sample was performed in duplicate.
X-ray diffraction
An isothermal crystallization was performed before analyses. Samples were heated and kept at 75 °C for 10 min, subsequently cooled at 10 °C for 1 min and kept at 19 °C to achieve crystallization. The X-ray diffraction was carried out with an Empyream diffractometer (Panalytical Almelo, The Netherlands), The diffractometer was equipped with a sealed Cu-X-ray tube, 0.04 rad primary and secondary Soller slits and a Ni-filter, with radiation Cu-Kα (k=1.541 Å, voltage 45 kV, current 40 mA, λ = 1.540). The simple was scanned from 1 to 40° in 2θ, with a step size of 0.026°. The oleogel samples were crystallized at 19 °C and placed on a rectangular sample holder to carry out the analysis. Each sample was performed in duplicate.
Experimental design and statistical analyses
Determination of the effects of each of the factors on onset temperature (Tonset) and crystallization temperature (Tc) was performed with response surface methodology using d-optimal design with three numerical factors: destilliertes monoglycerides (0 and 10%), beeswax concentrate (0 and 1%) and sorbitan monostearate (0 and 2%). Also, the categorical factor defined as the cooling rate of 3 and 5 °C for 1 min was considered. The experimental design consisted of 14 points for the model, five to determine unadjusted factors and 5 replicates. Analysis of variance (ANOVA) were carried out to evaluate the influence of the response variables of interesting, considering a significance level of 5%. The results were analyzed and processed using Desing Expert Software 8.0.5.
RESULTS AND DISCUSSION
The Analysis of Variance (Table 2), shows that the organogelator has a significant effect (P<0.05) on the Tc and Tonset of avocado oil and therefore on the oleoegel prepared, affecting the crystallization in the temperature range studied. Interactions among the factors of monoglyceride and SMS, as well as beeswax and SMS, also have a significant effect on the Tc and Tonset. On the other hand, there is no significant interaction among the factors of beeswax and cooling rate nor among SMS and cooling rate on the Tc and Tonset.
Effect of monoglycerides and beeswax on the crystallisation
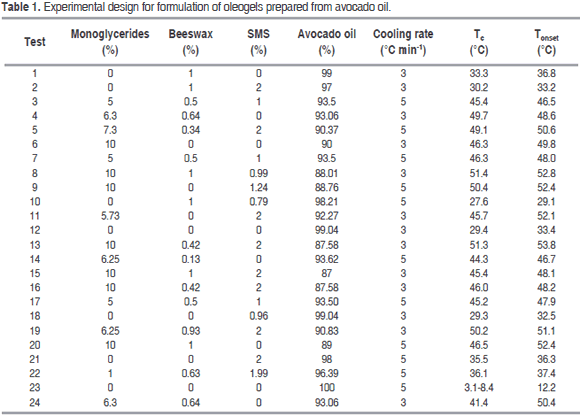
Figure 1 shows the thermal behavior of the oleogel prepared by monoglycerides 10% (Test 6), monoglycerides 6.3%-beeswax 0.64% (Test 24), as well as the thermal behavior of avocado oil. The formation of two structures crystallizing between 14 °C, and 47 °C can be observed. These temperatures are close to the crystallization temperatures shown by monoglycerides commercial grade, but with a smaller peak crystallization. The crystallization observed at elevated temperatures was related to the content of the saturated fatty acids in the monoglycerides and in the beeswax, and the crystallization located at a lower temperature was due to the presence of the unsaturated fatty acids.
It may also be observed that the Tonset and Tc (Table 2) are affected by the concentration of monoglycerides (P<0.05), probably due to the interaction between them and the TAGs from avocado oil. When the solid concentration is low (Test 24), the Tc and Tonset are moved to a lower temperature region, while crystallization enthalpy (DHc) decreases. Results similar to the above have been found in the crystallization study of milk fat, in fat blends having low and high melting points, in oleogels of palm with diacylglycerol and in oleogels from candelilla wax (Toro-Vazquez et al., 2009; Martini et al., 2008; Higaki et al., 2004; Tan and Che Man, 2002; Saberi et al., 2011; Foubert et al., 2004). The unsaturated fatty acids of avocado oil as oleic, linolenic and linoleic acid can affect the crystallization temperature, shifting the Tc and Tonset of the oleogel to a region of lower temperature. This phenomenon can be attributed to the steric effect of double bonds present in the TAGs of oil, which prevents the accommodation of monoglycerides chains, forming a less compact structure and a lower Tc and Tonset.
Given the high unsaturation of most TAGs present, the thermal behavior for avocado oil (Figure 1) showed a Tonset around 12.2 °C and a Tc at 8.4 and 3.1 °C, this result agrees with those found in studies of the crystallization of the oleogels of candelilla wax using safflower oil high in triolein (Toro-Vazquez et al., 2009). It was verified that the organogelators employed are capable of increasing the crystallization temperature of the avocado oil: the three organogels shift the crystallization to a zone of higher temperature, and this was dependent on the concentration of each one (P<0.05). This result agrees with those found in studies of the crystallization of the oleogels of candelilla wax, in the crystallization of milk fat and oleogels prepared with sorbitan monostearate (Toro-Vazquez et al., 2009; Martini et al., 2008; Murdan et al., 1999a; Hwang et al., 2011).
Effect of cooling rate, beeswax and SMS on the crystallisation
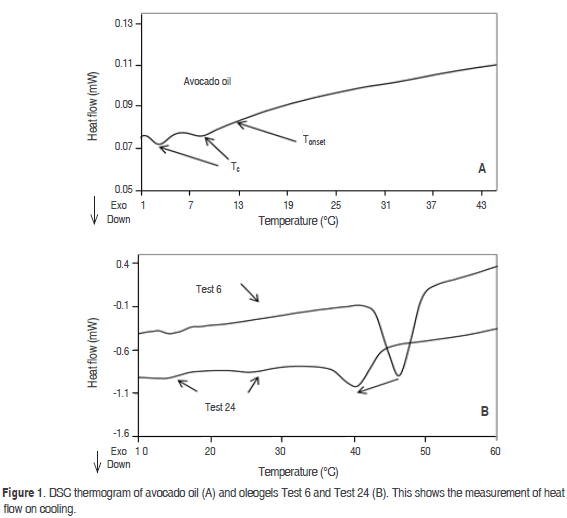
The increase of the Tc of avocado oil can be observed in figure 2. Oleogel (Test 2, 3 °C min-1) with SMS 2%-beeswax 1% has one crystallization peak at 30.2 °C. The crystallization occurred at a lower temperature than in the oleogel with beeswax 1% (Test 1, 3 °C min-1). The presence of SMS adversely affects the appearance of the Tc, this phenomenon was associated with the heterogenous nucleation of beeswax induced by the crystallization of SMS. When the cooling rate was increased in the Test 2 to 5 °C min-1, a decrease of its Tc and an increase in its enthalpy was observed, yielding values of 27.4 °C (Figure 2). In all of the oleogels a similar trend to reduce the Tc was observed when the cooling rate was performed at 5 °C min-1. A similar result was reported by Bouzidi and Narine (2012) in the crystallization of tristearoilglicerol and 1-palmitoyl-2,3-stearoyl glicerol.
Effect of cooling rate monodiglycerides on the crystallisation
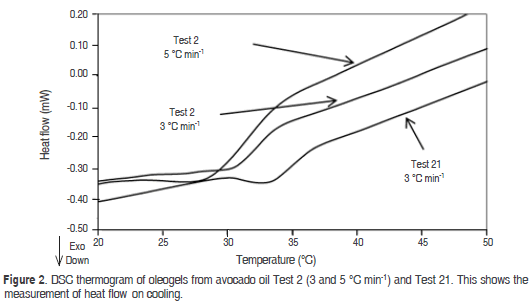
The oleogel shown in figure 3 revealed the presence of three exothermic peaks, probably caused by the crystallisation processes of α, b', b-forms. The first one is in the high temperature range between 40-54 °C and DHc 2.09 J·g-1 (Test 20.5 °C min-1), this peak can be caused by the presence of the b polymorph, attributed to the presence of a high concentration of trisaturated TAG. Another peak appears at 30 °C, which may be caused by the presence of the b' polymorph. The latter exothermic crystallisation peaks at 14.9 °C can be caused by the presence of the α polymorph, which crystallised at low temperatures, it can be probably due to the presence of unsaturated fatty acids. Likewise, there is a tendency to reduce the Tc when the cooling rate increases. This result agrees with that found in the oleogels of soybean oil and esters of waxes, and candelilla wax and tripalmitin preparations (Hwang et al., 2011; Toro-Vazquez et al., 2009). At a lower cooling rate, it is likely that the crystals tend to join more slowly and create smaller structures, which require less energy (enthalpy of crystallization) to crystallize. The opposite occurs with a high cooling rate, which forms larger crystals that require more energy to crystallize. This behavior was reported in the crystallization of palm oil and tripalmitin monoacylglycerols (Basso et al., 2010).
According to figure 3, the oleogel prepared with monoglycerides 10% (Test 6) shows two crystallization peaks. The first peak occurs at 47.3 °C with an enthalpy of 7.08 J·g-1. The Tc is lower compared to the one presented by monoglycerides (commercial grade). Another peak appears at 14.5 °C with an enthalpy of 0.22 J·g-1, which is close to that exhibited by the unsaturated components from monoglycerides (commercial grade). These exothermic crystallisation peaks can be caused by the presence of the b and α polymorphs respectively, similar results were found by Basso et al. (2010) in the study of crystallization of palm oil with tripalmitin and monoacylglycerols, it is shown that the addition of monoacylglycerols accelerated the crystallisation of palm oil by increasing the number of crystallisation seeds, reducing the size of the crystals formed and favouring the formation of b-crystals. The higher percentages of diglyceride promoted the crystallisation of the stable b polymorph (Saberi et al., 2011).
Effect of beeswax and SMS on the crystallisation
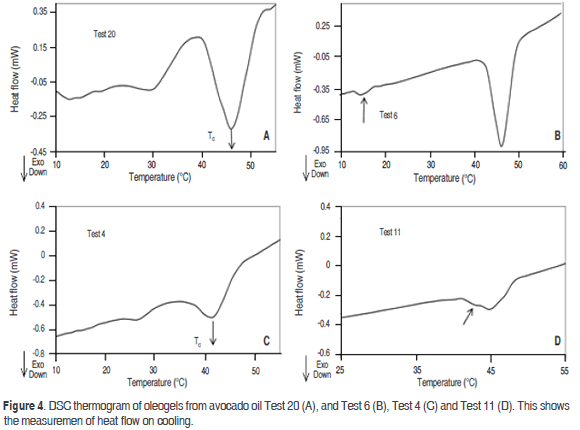
The combination of the organogelators of monodiglycerides 10% w/w-beeswax 1% w/w (Test 20) has three peaks of crystallization (Figure 4). The first one is in the high temperature range. It can be verified that in this zone, the increased concentration of beeswax from 0.64% w/w and monodiglycerides 6.3% (Test 4) to monodiglyceride 10%-beeswax 1% (Test 20) increases the solids content, thus increasing the Tc; this affects the shape and size of the peak. The bimodal melting profile is probably due to the formation of a recrystallized polymorphic form at that temperature. Similar results were found in oleogels of fully hydrogenated canola oil (Omonov et al., 2010). The mixture of SMS 2%-monodiglycerides 5.73% (Test 11), leads to the crystallization of avocado oil at about 46 °C with an enthalpy of 0.9 J·g-1. SMS affects the crystallization of the unsaturated components present in the oleogel, causing them to avoid crystallization in the region close to 14 °C, an area in which they normally crystallize, as shown in the thermogram of monodiglycerides 10% (Test 6). Sorbitan mono and tristearate have been shown to retard polymorphic transitions of saturated monoacid triglycerides into the more stable form (Jacome et al., 1989; Elisabettini et al., 1995). A near shoulder can also be observed at 42 °C (Test 11), probably due to the formation of structures formed by parallel chains saturated monodiglycerides and recrystallized SMS in this area. Generally, the presence of SMS affects the appearance of crystallization when mixed with monodiglycerides. Thus, in the high temperature zone, a tendency to decrease the Tc was observed. However, this can also be attributed to a lower concentration of monodiglycerides: the sample contains 5.73% monodiglycerides-2% SMS (Test 11) compared to 10% of the oleogel containing only monodiglycerides (Test 6).
X-ray diffraction Analysis
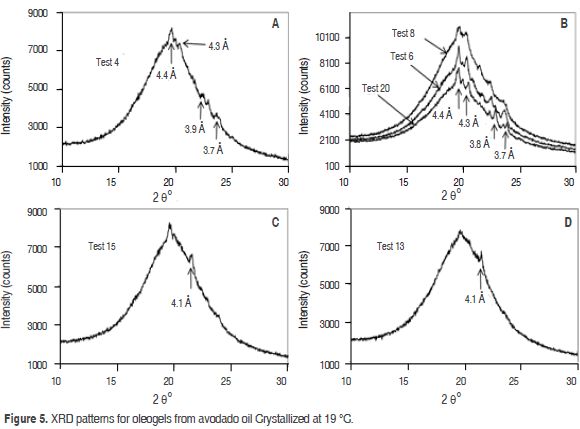
Figure 5 A,B shows the XRD patterns for oleogels produced (crystallized at 19 °C) from the combination of the three structuring agents (Tests 20, 8, 6 and 4). They are characterized by the absence of a strong signal at 4.2 A° (20.5° 2θ) that indicates the absence of a polymorphic form in alpha (α) configuration. Two peaks with medium intensity patterns 3.7 and 3.9 Å (22-23° 2θ), characteristic of the polymorphic form b' were found, although these patterns in Test 8 were not clear. On the other hand, signal around 4.6 Å indicates the transformation of the polymorphic forms b' to b, but it was not found, this indicates that the oleogels made from avocado oil (Tests 20, 6, 8 and 4) contain the b' form and that oleogel experienced no change in its structure under these conditions. The polymorphic form b' can be due to the presence of diglycerides in the mixture. A similar result was found by Saberi et al. (2011). In the study by X-ray diffraction of palm oil in blends with palm based diacylglycerol, it is shown that the polymorphic form b' exists at room temperature. This is the product of stearic acid content, which increases its stability. Avocado oil has 78% of unsaturated fatty acids, which necessarily affects the symmetry of the molecules of TAGs, forcing the folding of the chains and generating asymmetric structures. According to Timms (1984) these are responsible for the formation of polymorphic structures in the b' state. However, in several studies it has been established that the monoglycerides induce the formation of structures in the b state (Saber et al., 2011; Szydlowskaczerniak et al., 2005; Ghotra et al., 2002; Ribeiro et al., 2009; Basso et al., 2010).
Figure 5 C,D shows the XRD patterns for oleogels (crystallized at 19 °C) with SMS (Tests 13 and 15). One diffraction peak at 4.1 Å characteristic of the polymorphic form α were found. Similar results were found by Pernetti et al. (2007) in the study of crystallization of sunflower oil with lecithin and sorbitan tri-stearate, in comparison to other Tests (20, 6, 8 and 4) when the concentration of SMS was increased to 2% in the Test 13 and 15, a b'-destabilizing effect on oleogels from avocado oil was observed.
CONCLUSIONS
Beeswax, monodiglycerides and SMS, or combinations thereof, have the ability to increase the Tc and Tonset of avocado oil. The three organogelators shift the crystallization to a zone of higher temperature, and this is dependent on the concentration of each one. In all of the oleogels the tendency to decrease the Tc was observed when the crystallization process is performed at 5 °C min-1. At a higher cooling rate, the Tc is lower, and the crystallization enthalpy is higher. The XRD patterns for the oleogels produced from the three organogelators and crystallized isothermally at 19 °C, are characterized by the absence of the polymorphic form beta b. When the concentration of SMS was increased to 2 % a b'-destabilizing effect on oleogels made from avocado oil was observed.
ACKNOWLEDGMENTS
The authors wish to acknowledge to the Programa Generación de Conocimento, Secretaria de Productividad y Competitividad de la Gobernación de Antioquia. Medellín-Colombia, 2013 for the support of this study.
REFERENCES
- Basso R, Badan AP, Monise H, Gioielli L, Guaraldo L, Oliveira A and Grimaldi R. 2010. Tripalmitin and monoacylglycerols as modifiers in the crystallisation of palm oil. Food Chemistry 122(4): 1185-1192. doi: 10.1016/j.foodchem.2010.03.113
- Betancur D. 2011. Caracterización fisicoquímica del aguacate (Persea americana mill. cv. Hass) y estandarización del proceso de extracción del aceite. Tesis de Maestría en Ciencia y Tecnología de Alimentos. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia. Medellín. 56 p.
- Bot A, Den Adel R, Regkos C, Sawalha H, Venema P and Flöter E. 2011. Structuring in b-sitosterol+γ-oryzanol-based emulsion gels during various stages of a temperature cycle. Food Hydrocolloids 25(4): 639-46. doi: 10.1016/j.foodhyd.2010.07.026
- Bouzidi L and Narine SS. 2012. Relationships between molecular structure and kinetic and thermodynamic controls in lipid systems. Part III. Crystallization and phase behavior of 1-palmitoyl-2,3-stearoyl-sn-glycerol (PSS) and tristearoylglycerol (SSS) binary system. Chemistry and Physics of Lipids 165(1): 105-119. doi: 10.1016/j.chemphyslip.2011.11.004
- Da Pieve S, Calligaris S, Panozzo A, Arrighetti G and Nicoli MC. 2011. Effect of monoglyceride organogel structure on cod liver oil stability. Food Research International 44(9): 2978-2983. doi: 10.1016/j.foodres.2011.07.011
- Dassanayake LSK, Kodali DR and Ueno S. 2011. Formation of oleogels based on edible lipid materials. Current Opinion in Colloid and Interface Science 16(5): 432-439. doi: 10.1016/j.cocis. 2011.05.005
- Elisabettini P, Desmedt A, Gibon V and Durant F. 1995. Effect of sorbitan tristearate on the thermal and structural properties of monoacid triglycerides: influence of a "Cis" or "Trans" double bond. Europan Journal of Lipid Science and Technology 97(2): 65-69. doi: 10.1002/lipi.19950970206
- Foubert I, Vanhoutte B and Dewettinck K. 2004. Temperature and concentration dependent effect of partial glycerides on milk fat crystallization. European Journal of Lipid Science and Technology 106(8): 531-539. doi: 10.1002/ejlt.200400979
- Ghotra BS, Dyal SD and Narine S S. 2002. Lipid shortenings: a review. Food Research International 35(10): 1015-1048. doi: 10.1016/S0963-9969(02)00163-1
- Higaki K, Koyano T, Hachiya I and Sato K. 2004. In situ optical observation of microstructure of b-fat gel made of binary mixtures of high-melting and low-melting fats. Food Research International 37(1): 2-10. doi: 10.1016/j.foodres.2003.09.006
- Himawan C, Starov VM and Stapley AGF. 2006. Thermodynamic and kinetic aspects of fat crystallization. Advances in Colloid and Interface Science 122: 3-33. doi: 10.1016/j.cis.2006.06.016
- Hwang HS, Kim S, Singh M, Winkler-Moser JK and Liu SX. 2011. Organogel formation of soybean oil with waxes. Journal of the American Oil Chemists' Society 89(4): 639-647. doi: 10.1007/s11746-011-1953-2
- Jacome O, Aronhime J and Garti N. 1989. Polymorphic transitions of mixed triglycerides SOS in the presence of sorbitan monostearate. JAOCS 66(11): 1606-1613. doi: 10.1007/BF02636186
- Lupi F, Gabriele D, Facciolo D, Baldino N, Seta L and Cindio B. 2012. Effect of organogelator and fat source on rheological properties of olive oil-based organogels. Food Research International 46(1): 177-184. doi: 10.1016/j.foodres.2011.11.029
- Marangoni AG. 2002. Special issue of FRI-Crystallization, structure and functionality of fats. Food Research International 35(10): 907-908. doi: 10.1016/S0963-9969(02)00152-7
- Martini S, Carelli AA and Lee J. 2008. Effect of the addition of waxes on the crystallization behavior of anhydrous milk fat. Journal of the American Oil Chemists' Society 85(12): 1097-1104. doi: 10.1007/s11746-008-1310-2
- Morales-Rueda JA, Dibildox-Alvarado E, Toro-Vazquez J and Charo M. 2009. Rheological properties of candelilla wax and dotriacontane organogels measured with a True-Gap system. Journal of the America Oils Society 86(8): 765-772. doi: 10.1007/s11746-009-1414-3
- Murdan S, Gregoriadis G and Florence AT. 1999a. Inverse toroidal vesicles: precursors of tubules in sorbitan monostearate organogels. International Journal of Pharmaceutics 183(1): 47-49. doi: 10.1016/S0378-5173(99)00042-3
- Murdan S, Gregoriadis G and Florence AT. 1999b. Novel sorbitan monostearate organogels. Journal Pharmaceutical Sciences 88(6): 608-614. doi: 10.1021/js980342r
- Omonov T, Bouzidi L and Narine S. 2010. Quantification of oil binding capacity of structuring fats: A novel method and its application. Chemistry and Physics of Lipids 163(7): 728-740. doi: 10.1016/j.chemphyslip.2010.07.003
- Ortiz-Moreno A, Dorantes L, Galindez J and Guzman-Geronimo R. 2003. Effect of different extraction methods on fatty acids, volatile compounds, and physical and chemical properties of avocado (Persea americana Mill). Journal of Agricultural and Food Chemistry 51(8): 2216-2221. doi: 10.1021/jf0207934
- Pernetti M, Van KF, Flöter E and Bot A. 2007. Structuring of edible oils by alternatives to crystalline fat. Current Opinion in Colloid & Interface Science 12(4-5): 221-231. doi: 10.1016/j.cocis.2007.07.002
- Raut S, Singh S, Uplanchiwar V and Mishra V. 2011. Lecithin organogel: A unique micellar system for the delivery of bioactive agents in the treatment of skin aging. Acta Pharmaceutica Sinica B 2(1): 8-15. doi: 10.1016/j.apsb.2011.12.005
- Ribeiro APB, Basso RC, Grimaldi R, Gioielli LA, dos Santos AO, Cardoso LP and Gonçalves LAG. 2009. Influence of chemical interesterification on thermal behavior, microstructure, polymorphism and crystallization properties of canola oil and fully hydrogenated cottonseed oil blends. Food Research International 42(8): 1153-1162. doi: 10.1016/j.foodres.2009.05.016
- Saberi AH, Chin-Ping T and Oi-Ming L. 2011. Phase behavior of palm oil in blends with palm-based diacylglycerol. Journal of the American Oil Chemists' Society 88(12): 1857-1865. doi: 10.1007/s11746-011-1860-6
- Saberi AH, Lai OM and Toro-Vázquez JF. 2011. Crystallization kinetics of palm oil in blends with palm-based diacylglycerol. Food Research International 44(1): 425-435. doi: 10.1016/j.foodres.2010.09.029
- Sato K and Ueno S. 2011. Crystallization, transformation and microstructures of polymorphic fats in colloidal dispersion states. Current Opinion in Colloid & Interface Science 16(5): 384-390. doi: 10.1016/j.cocis.2011.06.004
- Szydlowskaczerniak A, Karlovits G, Lach M and Szlyk E. 2005. X-ray diffraction and differential scanning calorimetry studies of b'- b transitions in fat mixtures. Food Chemistry 92(1): 133-141. doi: 10.1016/j.foodchem.2004.07.010
- Tan C and Che Man Y. 2002. Differential scanning calorimetric analysis of palm oil, palm oil based products and coconut oil: effects of scanning rate variation. Food Chemistry 76(1): 89-102. doi: 10.1016/S0308-8146(01)00241-2
- Timms RE. 1984. Phase behaviour of fats and their mixtures. Progress in Lipid Research 23(1): 1-38. doi: 10.1016/0163-7827(84)90004-3
- Toro-Vazquez JF, Alonzo-Macias M, Dibildox-Alvarado E and Charó-Alonso MA. 2009. The effect of tripalmitin crystallization on the thermomechanical properties of Candelilla wax organogels. Food Biophysics 4(3): 199-212. doi: 10.1007/s11483-009-9118-7
- Toro-Vazquez JF, Morales-Rueda J, Mallia VA and Weiss RG. 2010. Relationship between molecular structure and thermo-mechanical properties of Candelilla wax and amides derived from (R)-12-Hydroxystearic acid as gelators of safflower oil. Food Biophysics 5(3): 193-202. doi: 10.1007/s11483-010-9159-y
References
Basso R, Badan AP, Monise H, Gioielli L, Guaraldo L, Oliveira A and Grimaldi R. 2010. Tripalmitin and monoacylglycerols as modifiers in the crystallisation of palm oil. Food Chemistry 122(4): 1185-1192. doi: 10.1016/j.foodchem.2010.03.113
Betancur D. 2011. Caracterización fisicoquímica del aguacate (Persea americana mill. cv. Hass) y estandarización del proceso de extracción del aceite. Tesis de Maestría en Ciencia y Tecnología de Alimentos. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia. Medellín. 56 p.
Bouzidi L and Narine SS. 2012. Relationships between molecular structure and kinetic and thermodynamic controls in lipid systems. Part III. Crystallization and phase behavior of 1-palmitoyl-2,3-stearoyl-sn-glycerol (PSS) and tristearoylglycerol (SSS) binary system. Chemistry and Physics of Lipids 165(1): 105-119. doi: 10.1016/j.chemphyslip.2011.11.004
Da Pieve S, Calligaris S, Panozzo A, Arrighetti G and Nicoli MC. 2011. Effect of monoglyceride organogel structure on cod liver oil stability. Food Research International 44(9): 2978-2983. doi: 10.1016/j.foodres.2011.07.011
Dassanayake LSK, Kodali DR and Ueno S. 2011. Formation of oleogels based on edible lipid materials. Current Opinion in Colloid and Interface Science 16(5): 432-439. doi: 10.1016/j.cocis. 2011.05.005
Elisabettini P, Desmedt A, Gibon V and Durant F. 1995. Effect of sorbitan tristearate on the thermal and structural properties of monoacid triglycerides: influence of a "Cis" or "Trans" double bond. Europan Journal of Lipid Science and Technology 97(2): 65-69. doi: 10.1002/lipi.19950970206
Foubert I, Vanhoutte B and Dewettinck K. 2004. Temperature and concentration dependent effect of partial glycerides on milk fat crystallization. European Journal of Lipid Science and Technology 106(8): 531-539. doi: 10.1002/ejlt.200400979
Ghotra BS, Dyal SD and Narine S S. 2002. Lipid shortenings: a review. Food Research International 35(10): 1015-1048. doi: 10.1016/S0963-9969(02)00163-1
Higaki K, Koyano T, Hachiya I and Sato K. 2004. In situ optical observation of microstructure of b-fat gel made of binary mixtures of high-melting and low-melting fats. Food Research International 37(1): 2-10. doi: 10.1016/j.foodres.2003.09.006
Himawan C, Starov VM and Stapley AGF. 2006. Thermodynamic and kinetic aspects of fat crystallization. Advances in Colloid and Interface Science 122: 3-33. doi: 10.1016/j.cis.2006.06.016
Hwang HS, Kim S, Singh M, Winkler-Moser JK and Liu SX. 2011. Organogel formation of soybean oil with waxes. Journal of the American Oil Chemists' Society 89(4): 639-647. doi: 10.1007/s11746-011-1953-2
Jacome O, Aronhime J and Garti N. 1989. Polymorphic transitions of mixed triglycerides SOS in the presence of sorbitan monostearate. JAOCS 66(11): 1606-1613. doi: 10.1007/BF02636186
Lupi F, Gabriele D, Facciolo D, Baldino N, Seta L and Cindio B. 2012. Effect of organogelator and fat source on rheological properties of olive oil-based organogels. Food Research International 46(1): 177-184. doi: 10.1016/j.foodres.2011.11.029
Marangoni AG. 2002. Special issue of FRI-Crystallization, structure and functionality of fats. Food Research International 35(10): 907-908. doi: 10.1016/S0963-9969(02)00152-7
Martini S, Carelli AA and Lee J. 2008. Effect of the addition of waxes on the crystallization behavior of anhydrous milk fat. Journal of the American Oil Chemists' Society 85(12): 1097-1104. doi: 10.1007/s11746-008-1310-2
Morales-Rueda JA, Dibildox-Alvarado E, Toro-Vazquez J and Charo M. 2009. Rheological properties of candelilla wax and dotriacontane organogels measured with a True-Gap system. Journal of the America Oils Society 86(8): 765-772. doi: 10.1007/s11746-009-1414-3
Murdan S, Gregoriadis G and Florence AT. 1999a. Inverse toroidal vesicles: precursors of tubules in sorbitan monostearate organogels. International Journal of Pharmaceutics 183(1): 47-49. doi: 10.1016/S0378-5173(99)00042-3
Murdan S, Gregoriadis G and Florence AT. 1999b. Novel sorbitan monostearate organogels. Journal Pharmaceutical Sciences 88(6): 608-614. doi: 10.1021/js980342r
Omonov T, Bouzidi L and Narine S. 2010. Quantification of oil binding capacity of structuring fats: A novel method and its application. Chemistry and Physics of Lipids 163(7): 728-740. doi: 10.1016/j.chemphyslip.2010.07.003
Ortiz-Moreno A, Dorantes L, Galindez J and Guzman-Geronimo R. 2003. Effect of different extraction methods on fatty acids, volatile compounds, and physical and chemical properties of avocado (Persea americana Mill). Journal of Agricultural and Food Chemistry 51(8): 2216-2221. doi: 10.1021/jf0207934
Pernetti M, Van KF, Flöter E and Bot A. 2007. Structuring of edible oils by alternatives to crystalline fat. Current Opinion in Colloid & Interface Science 12(4-5): 221-231. doi: 10.1016/j.cocis.2007.07.002
Raut S, Singh S, Uplanchiwar V and Mishra V. 2011. Lecithin organogel: A unique micellar system for the delivery of bioactive agents in the treatment of skin aging. Acta Pharmaceutica Sinica B 2(1): 8-15. doi: 10.1016/j.apsb.2011.12.005
Ribeiro APB, Basso RC, Grimaldi R, Gioielli LA, dos Santos AO, Cardoso LP and Gonçalves LAG. 2009. Influence of chemical interesterification on thermal behavior, microstructure, polymorphism and crystallization properties of canola oil and fully hydrogenated cottonseed oil blends. Food Research International 42(8): 1153-1162. doi: 10.1016/j.foodres.2009.05.016
Saberi AH, Chin-Ping T and Oi-Ming L. 2011. Phase behavior of palm oil in blends with palm-based diacylglycerol. Journal of the American Oil Chemists' Society 88(12): 1857-1865. doi: 10.1007/s11746-011-1860-6
Saberi AH, Lai OM and Toro-Vázquez JF. 2011. Crystallization kinetics of palm oil in blends with palm-based diacylglycerol. Food Research International 44(1): 425-435. doi: 10.1016/j.foodres.2010.09.029
Sato K and Ueno S. 2011. Crystallization, transformation and microstructures of polymorphic fats in colloidal dispersion states. Current Opinion in Colloid & Interface Science 16(5): 384-390. doi: 10.1016/j.cocis.2011.06.004
Szydlowskaczerniak A, Karlovits G, Lach M and Szlyk E. 2005. X-ray diffraction and differential scanning calorimetry studies of b'- b transitions in fat mixtures. Food Chemistry 92(1): 133-141. doi: 10.1016/j.foodchem.2004.07.010
Tan C and Che Man Y. 2002. Differential scanning calorimetric analysis of palm oil, palm oil based products and coconut oil: effects of scanning rate variation. Food Chemistry 76(1): 89-102. doi: 10.1016/S0308-8146(01)00241-2
Timms RE. 1984. Phase behaviour of fats and their mixtures. Progress in Lipid Research 23(1): 1-38. doi: 10.1016/0163-7827(84)90004-3
Toro-Vazquez JF, Alonzo-Macias M, Dibildox-Alvarado E and Charó-Alonso MA. 2009. The effect of tripalmitin crystallization on the thermomechanical properties of Candelilla wax organogels. Food Biophysics 4(3): 199-212. doi: 10.1007/s11483-009-9118-7
Toro-Vazquez JF, Morales-Rueda J, Mallia VA and Weiss RG. 2010. Relationship between molecular structure and thermo-mechanical properties of Candelilla wax and amides derived from (R)-12-Hydroxystearic acid as gelators of safflower oil. Food Biophysics 5(3): 193-202. doi: 10.1007/s11483-010-9159-y
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Sheah Yee Ghan, Lee Fong Siow, Chin Ping Tan, Kok Whye Cheong, Yin Yin Thoo. (2020). Influence of Soya Lecithin, Sorbitan and Glyceryl Monostearate on Physicochemical Properties of Organogels. Food Biophysics, 15(3), p.386. https://doi.org/10.1007/s11483-020-09633-z.
2. Beatriz Mariel Ferrer-González, Norma Leticia Flores-Martínez, Alfonso Totosaus. (2021). Ethanolic Extracts from Agro-Industrial Co-Products Enhance Oxidative Stability of Candelilla Wax or Celluloses Derivatives Oleogels. Acta Universitatis Cibiniensis. Series E: Food Technology, 25(1), p.83. https://doi.org/10.2478/aucft-2021-0008.
3. Elahe Kamali, Mohammad Ali Sahari, Mohsen Barzegar, Hassan Ahmadi Gavlighi. (2019). Novel oleogel formulation based on amaranth oil: Physicochemical characterization. Food Science & Nutrition, 7(6), p.1986. https://doi.org/10.1002/fsn3.1018.
4. Andrés Rumayor-Escobar, Mariana Morales-de la Peña, Julián de la Rosa-Millán, Teresita Arredondo-Ochoa, Elena Dibildox-Alvarado, Viridiana Tejada-Ortigoza. (2023). Effect of high intensity ultrasound on soybean and avocado oleogels’ structure and stability. Food Structure, 36, p.100315. https://doi.org/10.1016/j.foostr.2023.100315.
5. Liyang Du, Shaoyang Li, Qinbo Jiang, Yaoqiang Tan, Yuanfa Liu, Zong Meng. (2021). Interfacial interaction of small molecular emulsifiers tea saponin and monoglyceride: Relationship to the formation and stabilization of emulsion gels. Food Hydrocolloids, 117, p.106737. https://doi.org/10.1016/j.foodhyd.2021.106737.
6. Sara M. Oliveira, Artur J. Martins, Pablo Fuciños, Miguel A. Cerqueira, Lorenzo M. Pastrana. (2023). Food additive manufacturing with lipid-based inks: Evaluation of phytosterol-lecithin oleogels. Journal of Food Engineering, 341, p.111317. https://doi.org/10.1016/j.jfoodeng.2022.111317.
7. Fayza Hussein Alhasan, Mostafa Mazaheri Tehrani, Mehdi Varidi. (2024). Producing superior oleofoams: Unraveling the impact of oil type, surfactant concentration, and production temperature on foam stability and functional characteristics. Food Chemistry: X, 21, p.101033. https://doi.org/10.1016/j.fochx.2023.101033.
8. Dilshad Qureshi, Akhila Nadikoppula, Biswaranjan Mohanty, Arfat Anis, Miguel Cerqueira, Mayank Varshney, Kunal Pal. (2021). Effect of carboxylated carbon nanotubes on physicochemical and drug release properties of oleogels. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 610, p.125695. https://doi.org/10.1016/j.colsurfa.2020.125695.
9. F. Delbecq, R. Nguyen, E. Van Hecke, C. Len. (2019). Design and physicochemical properties of long and stiff fatty low molecular weight oleogelators. Journal of Molecular Liquids, 295, p.111708. https://doi.org/10.1016/j.molliq.2019.111708.
10. Somaye Pakseresht, Mostafa Mazaheri Tehrani. (2022). Advances in Multi-component Supramolecular Oleogels- a Review. Food Reviews International, 38(4), p.760. https://doi.org/10.1080/87559129.2020.1742153.
11. Xiaochen Wang, Da Ma, Yingwei Liu, Ying Wang, Chaoying Qiu, Yong Wang. (2022). Physical properties of oleogels fabricated by the combination of diacylglycerols and monoacylglycerols. Journal of the American Oil Chemists' Society, 99(11), p.1007. https://doi.org/10.1002/aocs.12622.
12. Liyang Du, Qinbo Jiang, Shaoyang Li, Qi Zhou, Yaoqiang Tan, Zong Meng. (2021). Microstructure evolution and partial coalescence in the whipping process of oleofoams stabilized by monoglycerides. Food Hydrocolloids, 112, p.106245. https://doi.org/10.1016/j.foodhyd.2020.106245.
13. D. Knoth, R. W. Eckert, S. F. Hartmann, C. M. Keck. (2019). Nanocosmetics. , p.199. https://doi.org/10.1007/978-3-030-16573-4_10.
14. Anna Magri, Milena Petriccione, Miguel A. Cerqueira, Tomy J. Gutiérrez. (2020). Self-assembled lipids for food applications: A review. Advances in Colloid and Interface Science, 285, p.102279. https://doi.org/10.1016/j.cis.2020.102279.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2016 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.