Effect of probiotic strain addition on digestive organ growth and nutrient digestibility in growing pigs
Efecto de la adición de cepas probióticas sobre el crecimiento de órganos digestivos y la digestibilidad de nutrientes en cerdos en crecimiento.
DOI:
https://doi.org/10.15446/rfna.v69n2.59136Keywords:
Antibiotic, Weaning, Diarrhea, Digestion, Piglet (en)Antibiótico, Destete, Diarrea, Digestión, Lechón (es)
Los cerdos son sometidos a diferentes tipos de estrés y para prevenirlo, se han utilizado los antibióticos como promotores de crecimiento (APC), generando residuos en el producto final y microorganismos con resistencia a antibióticos en el medio ambiente y en humanos. Como alternativa al uso de APC, se han utilizado bacterias probióticas que aportan beneficios a la salud del animal. Este trabajo tuvo como objetivo determinar el efecto comparativo de la adición de cepas probióticas sobre el crecimiento de órganos digestivos y la digestibilidad de nutrientes en cerdos en crecimiento. Ochenta lechones destetados a los 21 días de edad fueron alimentados con dos dietas: dieta comercial con y sin la adición de antibiótico; a esta última se adicionaron los diferentes probióticos (Lactobacillus casei, Lactobacillus acidophilus o Enterococcus faecium) en el agua de bebida. Se sacrificaron 35 lechones escalonadamente los días 1, 15 y 30 posdestete, y se extrajeron órganos de importancia digestiva; además se tomaron muestras de heces por colección rectal los días 15, 30 y 45 posdestete para estimar los coeficientes de digestibilidad aparente de nutrientes (marcador indigestible). Se observó un aumento significativo en el peso y desarrollo de órganos digestivos, y en los porcentajes de digestibilidad de nutrientes, específicamente calcio y fósforo, al comparar animales que consumieron E. faecium con aquellos que consumieron antibiótico. La adición de cepas probióticas (especialmente E. faecium), puede ser considerada como una alternativa al uso de APC cuando son suministradas en dietas de cerdos en fases críticas de crecimiento (posdestete), ya que mejoran la digestibilidad de nutrientes de importancia económica y ambiental como calcio y fósforo, disminuyendo su liberación al medio ambiente.
DOI: https://doi.org/10.15446/rfna.v69n2.59136
Effect of probiotic strain addition on digestive organ growth and nutrient digestibility in growing pigs
Efecto de la adición de cepas probióticas sobre el crecimiento de órganos digestivos y la digestibilidad de nutrientes en cerdos en crecimiento.
Santiago Londoño Pérez1, Jean-Paul Lallès2,3 and Jaime Parra Suescún1
1 BIOGEM Research Group. Facultad de Ciencias Agrarias. Universidad Nacional de Colombia. AA 1779. Medellín, Colombia.
2 Département de Nutrition Humaine. Institut National de la Recherche Agronomique. 63122. Saint-Genès Champanelle, France.
3 Centre de Recherche en Nutrition Humaine. Ouest 44093. Nantes Cedex 1, France. <jeparrasu@unal.edu.co>
Received: February 29, 2016; Accepted: July 6, 2016
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

ABSTRACT
Pigs are exposed to different types of stress. The growth-promoting antibiotics (GPA) used to counteract this stress generate residues in the final product and antibiotic-resistant microorganisms to the environment and humans. As an alternative to GPA, probiotic bacteria have been used to provide health benefits to these animals. This study aimed to determine the comparitive effect of probiotic strain addition on digestive organ growth and nutrient digestibility in growing pigs. Eighty piglets weaned at 21 days were fed two diets: a commercial diet with and without antibiotic added. Different probiotics were added to the drinking water for pigs fed the antibiotic-free diets (Lactobacillus casei, Lactobacillus acidophilus or Enterococcus faecium). Thirty-five piglets were slaughtered sequentially at days 1, 15 and 30 post-weaning, and their digestive organs were extracted. Feces were also sampled by rectal collection at days 15, 30 and 45 post-weaning, in order to estimate apparent nutrient digestibility coefficients (indigestible marker). A significant increase was observed in the weight and development of digestive organs and in the nutrient digestibility percentages, especially for calcium and phosphorus, when comparing the animals that consumed E. faecium with those that consumed antibiotic. The addition of probiotic strains (especially E. faecium) can be considered as an alternative to the use of GPA when these strains are administered in pig diets during critical stages of their growth (post-weaning), since they improve the digestibility of economically and environmentally important nutrients including calcium and phosphorus, thus decreasing their release into the environment.
Key words: Antibiotic, Weaning, Diarrhea, Digestion, Piglet
RESUMEN
Los cerdos son sometidos a diferentes tipos de estrés y para prevenirlo, se han utilizado los antibióticos como promotores de crecimiento (APC), generando residuos en el producto final y microorganismos con resistencia a antibióticos en el medio ambiente y en humanos. Como alternativa al uso de APC, se han utilizado bacterias probióticas que aportan beneficios a la salud del animal. Este trabajo tuvo como objetivo determinar el efecto comparativo de la adición de cepas probióticas sobre el crecimiento de órganos digestivos y la digestibilidad de nutrientes en cerdos en crecimiento. Ochenta lechones destetados a los 21 días de edad fueron alimentados con dos dietas: dieta comercial con y sin la adición de antibiótico; a esta última se adicionaron los diferentes probióticos (Lactobacillus casei, Lactobacillus acidophilus o Enterococcus faecium) en el agua de bebida. Se sacrificaron 35 lechones escalonadamente los días 1, 15 y 30 posdestete, y se extrajeron órganos de importancia digestiva; además se tomaron muestras de heces por colección rectal los días 15, 30 y 45 posdestete para estimar los coeficientes de digestibilidad aparente de nutrientes (marcador indigestible). Se observó un aumento significativo en el peso y desarrollo de órganos digestivos, y en los porcentajes de digestibilidad de nutrientes, específicamente calcio y fósforo, al comparar animales que consumieron E. faecium con aquellos que consumieron antibiótico. La adición de cepas probióticas (especialmente E. faecium), puede ser considerada como una alternativa al uso de APC cuando son suministradas en dietas de cerdos en fases críticas de crecimiento (posdestete), ya que mejoran la digestibilidad de nutrientes de importancia económica y ambiental como calcio y fósforo, disminuyendo su liberación al medio ambiente.
Palabras claves: Antibiótico, Destete, Diarrea, Digestión, Lechón
Weaning is one of the most critical events in swine production, since during this stage the stress generated by abrupt separation from the mother, the change of the nature and composition of feeds from sow's milk to plant-based solid feed (Ciro et al., 2013), and the poor development of the gastrointestinal tract and immune system in piglets results in a disruption of the mucosa integrity (Ciro et al., 2016). All these events could contribute to significant alterations in size and structure of the gut and digestive organs, which interfere with its functional capacity, particularly for the processes of digestion and nutrient absorption (Gutierrez et al., 2012).
The consumption of a new solid diet during the post-weaning stage results in a drastic reduction in feed consumption (Lallès and David, 2011). This leads to drastic decreases in intestinal microbial populations (primarily Lactobacillus spp. and Streptococcus spp.) and increases in the number of coliform bacteria (especially E. coli) during the first week after weaning.
The use of live microorganisms has been considered as a way to decrease the negative effects caused by weaning, since positive effects on the health of the host have been reported as a result of this practice. These include improvements in intestinal microbial balance, reduction of diarrhea, stimulation of the immune system, and prevention of gastrointestinal infections (Lye et al., 2012).
The gut microbiota is a complex system that can have a significant impact on the immune status of the host, since beneficial microorganisms have the capacity to suppress pathogenic bacterial populations by creating an unsuitable environment for these pathogens and inducing intestinal immune responses. Additionally, beneficial microorganisms have the ability to modify the fermentation product profiles and to generate substances (bacteriocins) that inhibit the growth of pathogenic bacteria (Mallo et al., 2010). Moreover, probiotics have proved stimulating capacities on the secretion of some endogenous enzymes (aminopeptidases and dissacharidases), thus improving the digestive function of animals and promoting the development and functionality of the intestinal villi (Reyes et al., 2012).
For these reasons, the aim of this study was to add different probiotic strains to the feed and comparatively evaluate their effects on digestive organ growth and nutrient digestibility in weaned piglets.
MATERIALS AND METHODS
Ethical statement
All experimental procedures were conducted according to guidelines suggested by "The International Guiding Principles for Biomedical Research Involving Animals" (CIOMS, 2012), and approved by the Ethics Committee on Animal Experimentation of Universidad Nacional de Colombia, Medellín (CEMED-03 of May 7th, 2012).
Location
The fieldwork was conducted in the commercial farm "Caña Brava", and is located in the municipality of Gómez Plata (Antioquia, Colombia), at 1540 masl, with an average temperature of 21 °C, corresponding to a tropical lower-montane wet forest zone.
Animals
Eighty Duroc x Pietrain crossbred piglets (male and female) weaned at 21 d of age and with an approximate weight of 6 ± 0.5 kg were used, which were separated into groups of 8 during the post-weaning period. Each of the pen was provided with trough-type feeders, in a controlled temperature room (26 ± 3 °C). Water was accessible ad libitum throughout the experiment. The commercial diets provided in a pelleted form were added with vitamins, minerals, and lysine HCl and balanced in order to meet all of the minimal nutrition requirements proposed by the NRC (2012). Feed (g) were offered ad libitum to the piglets in each pen, in accordance with the dietary recommendations for the productive (growing) stage. Meanwhile, the drinking water containing the different probiotic strains was provided daily from day 1 post-weaning until the end of the experiment at day 45 post-weaning. No solid feed was given to the piglets during the suckling stage.
Installations and equipment
The pigs were kept in pen with concrete floors (1.5 x 3 m), which were disinfected and whitewashed prior to the arrival of the piglets. From day 0 to day 15 of the experiment, the pen were equipped with piglet house and wood-chip bedding, and in order to maintain a homogenous temperature, the corral was fitted with curtains. A digital scale was used to weigh the pigs and the feed provided.
Diets
The animals were fed with two diets: a commercial diet with and without antibiotic added. The different probiotics (L. casei, L. acidophilus or E. faecium) were administered in the drinking water of the animals that consumed the commercial diet without antibiotic, as follows:
Diet 1 Negative control (D1): Commercial feed without antibiotic, without supplementation with probiotic strains in the drinking water.
Diet 2 Positive control (D2): Commercial feed with antibiotic (zinc bacitracin, manufacturer's recommended dosage), without supplementation with probiotic strains in the drinking water.
Diet 3 (D3): Commercial feed without antibiotic, with supplementation with the commercial probiotic strain L. acidophilus in the drinking water.
Diet 4 (D4): Commercial feed without antibiotic, with supplementation with the commercial probiotic strain L. casei in the drinking water.
Diet 5 (D5): Commercial feed without antibiotic, with supplementation with the commercial probiotic strain E. faecium in the drinking water.
The quantity of probiotic added was based on the instructions for their preparation and addition provided by the manufacturer's recommendations. The inclusion of the probiotics in the drinking water was carried out by directly mixing a liter of water with 30 g of commercial sugar, thereby guaranteeing minimal populations of 108 CFU (Colony-forming units) with suitable viability, which was diluted into water to reach a final volume of 50 L, and evaluated through microbiological analyses. The animals receiving water without probiotics also received one liter of water with 30 g of sugar in final 50 L of water. The experimental diets were provided for 30 d starting at the day of weaning (age of 21 d).
Zootechnical parameters
The data used to calculate the zootechnical parameters were taken at days 15, 30 and 45 post-weaning. The quantity of feed provided and refused was recorded daily in order to calculate total feed consumption, feed conversion, and feed efficiency. Body weight (BW) was also recorded on these same days.
Feces sampling
Approximately five grams of fecal matter were taken directly from the rectum, using sterile plastic 10 x 15 cm bags with 10 mL 0.2 N HCl added. The content of each bag was emptied into a plastic container that was kept refrigerated at -20 °C (in order to prevent bacterial proliferation) until its homogenization and laboratory analysis.
Total nutrient digestibility
Total apparent nutrient digestibility was evaluated through the indirect method, with chromium oxide (0.3% per kg of feed) used as an inert marker. Both the previously mentioned ingredients and the total diet were analyzed to determine dry matter, crude protein, energy, ether extract, crude fiber, ash, calcium and phosphorus, according to the methods described in AOAC (2012). Chromium was analysed using the method of Fenton and Fenton (1979). Feces collection was performed at days 15, 30 and 45 post-weaning, and this procedure was carried out twice a day (at 8:00 and 16:00 h). The total content and digestibility coefficients of dry matter, crude protein, energy, ether extract, crude fiber, ash, phosphorus, and calcium were determined in these samples.
Apparent digestibility (AD) coefficients were calculated as follows:
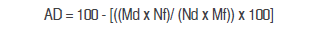
Where:
AD = Apparent digestibility (%)
Md = Concentration of the indicator in the diet (%)
Mf = Concentration of the indicator in the feces (%)
Nf = Concentration of the nutrient in the feces (%)
Nd = Concentration of the nutrient in the diet (%)
Animal euthanasia and organ sampling
During the experiment phase, euthanasia was carried out humanly on the 35 piglets in the following manner: on the initial day, or day 1 (day of weaning), 5 piglets were slaughtered randomly, which represented the reference group in order to verify the general state of health and the macroscopic evaluation of the state of the organs of the animals before providing the experimental diets; and day 15 and 30 post-weaning, 3 piglets were slaughtered randomly for each treatment, performing euthanasia to 30 piglets. All piglets were slaughtered 2.5 h after the last feed provision. The animals were sedated with the neuroleptic stresnil® (Azaperona) intramuscularly and were subsequently subjected to Nitrox® inhalation.
After the slaughter, the pigs were placed in supine position, the abdominal region was dissected, and the stomach, liver, pancreas, spleen, cecum, colon, and small intestine (from the pyloric valve to the ileocecal valve) were extracted completely (Segalés and Domingo, 2003). The intestine was aligned and measured on a table without any type of tension. Once the organs were extracted, each removed portion was washed with cold saline solution for eliminating digest contents (Reis et al., 2005). The organs were then weighed in order to determine digestive organ development.
Statistical analysis
Zootechnical parameters and total nutrient digestibility
A randomized block design was used (two blocks) with an arrangement of repeated measures (Steel and Torrie, 1997). The animals were blocked by initial weight. Each animal was assigned to one of five experimental diets (commercial feed: without antibiotic or probiotic, with antibiotic, and with L. casei, L. acidophilus or E. faecium added). Each treatment had a total of two replicates and eight animals per replicate. Statistical analysis was performed using the GLM procedure of SAS® (2007). The differences among treatments and periods were determined by least-squares (LS) means; additionally, Duncan's test was used to detect significance (P<0.05) among the means.
Digestive organ weights and total apparent digestibility coefficients
The experiment was carried out using a randomized block design with a split plot arrangement. The animals were blocked by initial weight. Each animal was assigned to one of 15 treatments (five experimental diets and three evaluation periods). Each treatment had a total of three replicates. Statistical analysis was performed using the GLM procedure of SAS® (2007). The differences between treatment means were determined by least-squares and analyzed by ANOVA. Duncan's test (P<0.05) was used to compare the averages among treatments.
RESULTS AND DISCUSSION
In general, the pigs that consumed the different diets showed a good health estatus and did not exhibit any signs of illness that would necessitate their withdrawal and/or immediate slaughter. In this experiment, no statistical interaction was observed between the dietary treatments and the weaning periods for any of the studied variables; therefore it was unnecessary to analyze those two factors independently.
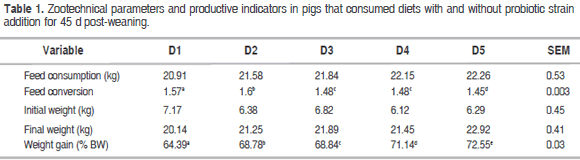
Table 1 presents the results for the zootechnical parameters evaluated in pigs that consumed diets with and without different probiotic strains added for 45 d post-weaning. For the feed consumption variable, no statistical differences were found among each of diets studied (P>0.05). For the variables of conversion and weight gain (%LW, live weight), statistical differences were found between diets. There was a statistical difference between D1 and D2 (P<0.05), with D2 showing the highest results. However, when D2 was compared with the diets supplemented with probiotics (D3, D4 and D5), D5 showed the best results for both variables.
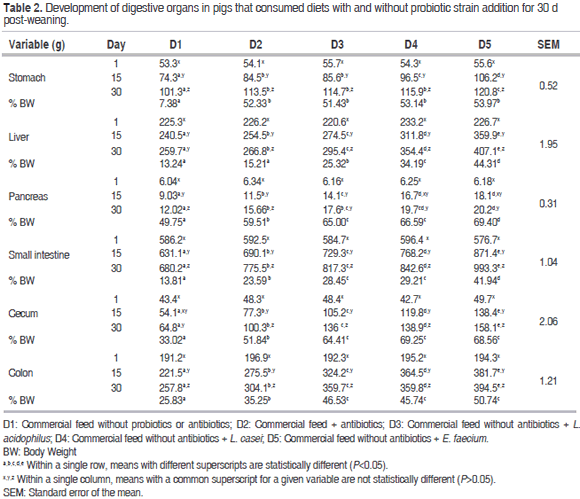
For the growth of the digestive organs variable (g and % BW), statistically significant differences were observed among diets within each of the sampling periods (15 and 30) (P<0.05). A statistically significant difference was observed (P<0.05) between the D1 and D2 diets, with D2 presenting better results. When compared with the diets containing probiotic strains, the organs evaluated in the animals that consumed D3, D4 and D5 weighed more than those that consumed D2. However, this was not observed for the stomach since there was no significant difference (P<0.05) between the D2 and D3 treatments. Table 2 thus, shows that the results for the animals that consumed feed with the E. faecium probiotic were superior to the others.
For the same variables under study (Table 2), there were statistically significant differences among the different sampling days within each of the diets (P<0.05), with day 30 presenting the highest values for each diet studied.
For fecal digestibility of nutritional compounds (Table 3), there was a significant difference (P<0.05) between the diets within each of the sampling periods (15, 30 and 45). Significant differences were observed (P<0.05) between the D1 and D2 treatments, with the exception of crude protein on day 45 of sampling, and energy on days 30 and 45 of sampling. A significant difference (P<0.05) was found when the digestibility coefficients obtained for the nutritional compounds in the diets with probiotic strains were compared with the D2 treatment, except for dry matter in D2 and D3 between these treatments (Table 3). Additionally, when nutrient digestibility coefficients were compared among the diets with probiotics (D3, D4 and D5), the diet with the probiotic E. faecium showed the highest (P<0.05, Table 3). For nutrient digestibility (Table 3), statistically significant differences were observed among the different sampling days within each of the diets (P<0.05), with the highest values occurring on day 45 for each diet studied.
Mallo et al. (2010) found that the addition of the E. faecium probiotic CECT 4515 strain to the diet of weaned piglets impacted the gastrointestinal microbiota by promoting lactic acid bacteria (LAB) growth in the gastrointestinal tract, thus improving growth and feed conversion. A recent study showed that this probiotic significantly increased the number of lactobacilli in the feces of sows and their piglets (Starke et al., 2013). A positive interaction was thus observed between this specific probiotic and the lactobacilli, which could be a mode of action explaining its beneficial effects. This agrees with the findings of the present experiment in which the pigs that consumed L. acidophilus and E. faecium had higher feed conversion ratios at the end of the experiment. The consumption of probiotic strains impacted pig body weight since higher weight gain was observed in the piglets that consumed E. faecium. This confirms previous studies reporting that the action of probiotics in piglets notably improved many zootechnical indices in the pigs, including weight gain, feed conversion ratio, feed digestibility, and offspring survival rate (Yu et al., 2004; Yang et al., 2015).
According to Kang et al. (2010), dietary changes could alter the microbial equilibrium of the gastrointestinal tract, which increases the risk of pathogen colonization of the gut. The gut microflora provide a barrier against pathogens since many species contribute to antibacterial defenses through the production of bacteriocins or defensins (Turroni et al., 2008), the reduction of luminal pH, the systemic immune response and the reinforcement of the nonspecific intestinal barrier (Ng et al., 2009). Additionally, various components of gut microflora play a crucial role in the postnatal development of the immune system. During the initial post-natal period, intestinal microorganisms stimulate the development of local and systemic immunity and, over time they regulate the maintainance mechanisms of mucosal immunity (Scholz-Ahrens et al., 2007; Tlaskalova-Hogenova et al., 2011).
Unequivocal evidence that the gut microbiota is essential for life and metabolism is seen in the fact that mammals raised germ-free, which do not acquire their normal gut microbiota at birth (LAB), tend to exhibit abnormal body development with an atrophic intestinal wall and altered motility; reduced metabolism; low weights of heart, lung and liver; low cardiac output; low body temperature; high blood cholesterol levels; and an immature immune system with low levels of immunoglobulins (Macpherson et al., 2001; Yang et al., 2015). These findings are in agreement with the data obtained in the present study in which the animals consuming diets supplemented with probiotics, particularly E. faecium presented the greatest organ growth and development.
At a nutritional level, lactobacilli possess the enzymes b-galactosidase and lactic dehydrogenase, which produce lactic acid from lactose. This could additionally promote the digestibility of the different compounds in milk; improve the use of calcium, phosphorus and iron; and increase vitamin synthesis (Tannock, 2005). This was confirmed in the present study since the animals consuming diets with probiotics presented higher calcium and phosphorus digestibility coefficients; this was highest with E. faecium strain.
In this study, we observed that the animals consuming different probiotic strains exhibited improved nutrient digestion, particularly for energy, protein and phosphorus, which are considered the most costly nutrients in swine nutrition. Fiber digestibility was also improved, in agreement with the findings of DiBaise et al. (2008). In that study, the authors proposed that gut microbiota lactic acid contributes to digestion processes by transforming dietary fiber or mucopolysaccharides into simple sugars, short chain fatty acids, and other absorbable nutrients. These LAB promote the production of vitamins K, B12 and folic acid; the recirculation of bile acids; the transformation of potential carcinogens, including the N-nitroso compound and heterocyclic amines; and the activation of some bioactive compounds, including phytoestrogens.
Due to the aforementioned reasons, the gut microbiota are increasingly considered as a symbiotic partner for health maintenance. This could explain the results obtained in this experiment, in which greater nutrient digestibility was observed in animals consuming feed with probiotic strains, particularly E. faecium.
For Reyes et al. (2012), one of the most important results of using probiotics, especially E. faecium, is the displacement of pathogenic gut flora towards LAB groups, since these bacteria reduce intestinal pH, thus stimulating the production of endogenous pepsin and improving the digestibility of dietary protein.
Giang et al. (2010) found that feeding diets supplemented with a probiotic lactobacillus complex to piglets for two weeks post-weaning improved animal growth and nutrient digestibility. Additionally, there was a reduction in the incidence of diarrhea during the period evaluated. This agrees with the present study.
CONCLUSIONS
The information obtained in this study suggest that administering probiotics, especially Enterococcus faecium, to growing pigs could be an alternative to using growth-promoting antibiotic, since they improve nutrient digestibility. This is evidenced in greater weight gain and development of digestive organs.
REFERENCES
AOAC. 2012. Official Methods of Analysis. Association of Official Analytical Chemists. Arlington. 19th edition. Arlington, Virginia (USA).
CIOMS. 2012. Council For International Organization Of Medical Sciences And The International Council For Laboratory Animal Science. International guiding principles for biomedical research involving animals. Disponible en: http://grants.nih.gov/grants/olaw/Guiding_Principles_2012.pdf
Ciro JA, López HA and Parra SJE. 2013. Expresión molecular de la vilina en yeyuno de lechones posdestete que consumieron LPS de E. coli. Revista CES Medicina Veterinaria y Zootecnia 8(2): 32-41. doi: 10.15446/rfmvz.v61n2.44677
Ciro JA, López HA and Parra SJE. 2016. The probiotic Enterococcus faecium modifies the intestinal morphometric parameters in weaning piglets. Revista Facultad Nacional de Agronomia 69(1): 1-10. doi: 10.15446/rfna.v69n1.54748
DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA and Rittmann BE. 2008. Gut microbiota and its possible relationship with obesity. Mayo Clinic Proceedings 83(4): 460-469. doi: 10.4065/83.4.460
Fenton T and Fenton M. 1979. An improved procedure for determination of chromic oxide in feed and feces. Canadian Journal Science 59: 631-634. doi: 10.4141/cjas79-081
Giang HH, Viet TQ, Ogle B and Lindberg JE. 2010. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livestock Science 129: 95-103. doi: 10.1016/j.livsci.2010.01.010
Gutierrez VC, Román OY, Peláez JC, Ciro GJ, López HA and Parra SJE. 2012. Efecto de la adición ex vivo del Lipopolisacárido de Escherichia coli sobre la absorción de Lisina en cerdos destete. Revista Facultad Nacional Agronomia 65: 6447-6457.
Kang P, Toms D, Yin Y, Cheung Q, Gong J, De Lange K and Li J. 2010. Epidermal growth factor-expressing Lactococcus lactis enhances intestinal development of early-weaned pigs. The Journal of Nutrition 140: 806-811. doi: 10.3945/jn.109.114173
Lallès JP and David JC. 2011. Fasting and refeeding modulate the expression of stress proteins along the gastro-intestinal tract of weaned pigs. Journal of Animal Physiology and Animal Nutrition 95: 478-488. doi: 10.1111/j.1439-0396.2010.01075.x
Lye H, Khoo BY, Karim AA, Rusul G and Liong MT. 2012. Growth properties and cholesterol removal ability of electroporated Lactobacillus acidophilus BT 1088. Journal of Microbiology and Biotechnology 7: 981-989. doi: 10.4014/jmb.1201.12073
Macpherson A, Hunziker L, McCoy K and Lamarre A. 2001. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes and Infection 3(12): 1021-1035. doi: 10.1016/S1286-4579(01)01460-5
Mallo J, Rioperez J and Honrubia P. 2010. The addition of Enterococcus faecium to diet improves piglet's intestinal microbiota and performance. Livestock Science 133: 176-178. doi: 10.1016/j.livsci.2010.06.057
Ng S, Hart AL, Kamm MA, Stagg AJ and Knight SC. 2009. Mechanisms of action of probiotics: recent advances. Inflammatory Bowel Diseases 15: 300-310. doi: 10.1002/ibd.20602.
NRC. 2012. National Research Council. The Nutrient Requirements of Swine. Eighth revised edition. National Academy Press, Washington, DC, USA.
Reis S, Guerrero C, Aguilera B and Mariscal L. 2005. Efecto de diferentes cereales sobre la morfología intestinal de lechones recién destetados. Técnica Pecuaria México 43: 309-321.
Reyes VI, Figueroa JL, Cobos MA, Sánchez-Torres MT, Zamora V and Cordero JL. 2012. Probiotico (Enterococcus Faecium) adicionado a dietas estandar y con baja proteina para cerdos en engorda. Archivos de Zootecnia 61: 236. doi: 10.4321/S0004-05922012000400011
SAS®. 2007. SAS/STAT User's Guide. Institute Inc. Statistical Analysis Systems Institute. Version 9. First edition. Cary, NC: SAS Institute Inc.
Scholz-Ahrens K, Ade P, Marten B, Weber P, Timm W, Açil Y, Glüer CC and Schrezenmeir J. 2007. Prebiotics, Probiotics, and Synbiotics affect mineral absorption, bone mineral content, and bone structure. Journal of Nutrition 137: 838s-846s.
Segalés J y Domingo M. 2003. La necropsia en el ganado porcino, diagnóstico anatomopatológico y toma de muestras. Boehringer Ingelheim, Madrid, España). pp. 10-14.
Starke I, Pieper R, Neumann K, Zentek J and Vahjen W. 2013. Individual responses of mother sows to a probiotic Enterococcus faecium strain lead to different microbiota composition in their offspring. Beneficial Microbes 4(4): 345-356. doi: 10.3920/BM2013.0021.
Steel RG, Torrie JH. 1997. Principles and procedures of statistics: a biometrical approach. Third edition. McGraw-Hill, New York. 672 p.
Tannock G. 2005. Probiotics y prebiotics: scientific aspects. Caister Academic Press, Norfolk, Reino Unido. pp. 25-49.
Tlaskalova-Hogenova H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Srůtková D, Zídek Z, Schwarzer M, Drastich P and Funda DP. 2011. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cellular and Molecular Immunology 8(2): 110-120. doi: 10.1038/cmi.2010.67
Turroni F, Ribbera A, Foroni E, van Sinderen D and Ventura M. 2008. Human gut microbiota and bifidobacteria: from composition to functionality. Antonie van Leeuwenhoek 94: 35-50. doi: 10.1007/s10482-008-9232-4.
Yang F, Hou C, Zeng X and Qiao S. 2015. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens 4: 34-45. doi: 10.3390/pathogens4010034
Yu I, Ju Ch-Ch, Lin J, Wu HL and Yen HT. 2004. Effects of probiotics and selenium combination on the immune and blood cholesterol concentration of pigs. Journal of Animal and Feed Science 13: 625-634.
References
AOAC. 2012. Official Methods of Analysis. Association of Official Analytical Chemists. Arlington. 19th edition. Arlington, Virginia (USA).
CIOMS. 2012. Council For International Organization Of Medical Sciences And The International Council For Laboratory Animal Science. International guiding principles for biomedical research involving animals. Disponible en: http://grants.nih.gov/grants/olaw/Guiding_Principles_2012.pdf
Ciro JA, López HA and Parra SJE. 2013. Expresión molecular de la vilina en yeyuno de lechones posdestete que consumieron LPS de E. coli. Revista CES Medicina Veterinaria y Zootecnia 8(2): 32-41. doi: 10.15446/rfmvz.v61n2.44677
Ciro JA, López HA and Parra SJE. 2016. The probiotic Enterococcus faecium modifies the intestinal morphometric parameters in weaning piglets. Revista Facultad Nacional de Agronomia 69(1): 1-10. doi: 10.15446/rfna.v69n1.54748
DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA and Rittmann BE. 2008. Gut microbiota and its possible relationship with obesity. Mayo Clinic Proceedings 83(4): 460-469. doi: 10.4065/83.4.460
Fenton T and Fenton M. 1979. An improved procedure for determination of chromic oxide in feed and feces. Canadian Journal Science 59: 631-634. doi: 10.4141/cjas79-081
Giang HH, Viet TQ, Ogle B and Lindberg JE. 2010. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livestock Science 129: 95-103. doi: 10.1016/j.livsci.2010.01.010
Gutierrez VC, Román OY, Peláez JC, Ciro GJ, López HA and Parra SJE. 2012. Efecto de la adición ex vivo del Lipopolisacárido de Escherichia coli sobre la absorción de Lisina en cerdos destete. Revista Facultad Nacional Agronomia 65: 6447-6457.
Kang P, Toms D, Yin Y, Cheung Q, Gong J, De Lange K and Li J. 2010. Epidermal growth factor-expressing Lactococcus lactis enhances intestinal development of early-weaned pigs. The Journal of Nutrition 140: 806-811. doi: 10.3945/jn.109.114173
Lallès JP and David JC. 2011. Fasting and refeeding modulate the expression of stress proteins along the gastro-intestinal tract of weaned pigs. Journal of Animal Physiology and Animal Nutrition 95: 478-488. doi: 10.1111/j.1439-0396.2010.01075.x
Lye H, Khoo BY, Karim AA, Rusul G and Liong MT. 2012. Growth properties and cholesterol removal ability of electroporated Lactobacillus acidophilus BT 1088. Journal of Microbiology and Biotechnology 7: 981-989. doi: 10.4014/jmb.1201.12073
Macpherson A, Hunziker L, McCoy K and Lamarre A. 2001. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes and Infection 3(12): 1021-1035. doi: 10.1016/S1286-4579(01)01460-5
Mallo J, Rioperez J and Honrubia P. 2010. The addition of Enterococcus faecium to diet improves piglet's intestinal microbiota and performance. Livestock Science 133: 176-178. doi: 10.1016/j.livsci.2010.06.057
Ng S, Hart AL, Kamm MA, Stagg AJ and Knight SC. 2009. Mechanisms of action of probiotics: recent advances. Inflammatory Bowel Diseases 15: 300-310. doi: 10.1002/ibd.20602.
NRC. 2012. National Research Council. The Nutrient Requirements of Swine. Eighth revised edition. National Academy Press, Washington, DC, USA.
Reis S, Guerrero C, Aguilera B and Mariscal L. 2005. Efecto de diferentes cereales sobre la morfología intestinal de lechones recién destetados. Técnica Pecuaria México 43: 309-321.
Reyes VI, Figueroa JL, Cobos MA, Sánchez-Torres MT, Zamora V and Cordero JL. 2012. Probiotico (Enterococcus Faecium) adicionado a dietas estandar y con baja proteina para cerdos en engorda. Archivos de Zootecnia 61: 236. doi: 10.4321/S0004-05922012000400011
SAS®. 2007. SAS/STAT User's Guide. Institute Inc. Statistical Analysis Systems Institute. Version 9. First edition. Cary, NC: SAS Institute Inc.
Scholz-Ahrens K, Ade P, Marten B, Weber P, Timm W, Açil Y, Glüer CC and Schrezenmeir J. 2007. Prebiotics, Probiotics, and Synbiotics affect mineral absorption, bone mineral content, and bone structure. Journal of Nutrition 137: 838s-846s.
Segalés J y Domingo M. 2003. La necropsia en el ganado porcino, diagnóstico anatomopatológico y toma de muestras. Boehringer Ingelheim, Madrid, España). pp. 10-14.
Starke I, Pieper R, Neumann K, Zentek J and Vahjen W. 2013. Individual responses of mother sows to a probiotic Enterococcus faecium strain lead to different microbiota composition in their offspring. Beneficial Microbes 4(4): 345-356. doi: 10.3920/BM2013.0021.
Steel RG, Torrie JH. 1997. Principles and procedures of statistics: a biometrical approach. Third edition. McGraw-Hill, New York. 672 p.
Tannock G. 2005. Probiotics y prebiotics: scientific aspects. Caister Academic Press, Norfolk, Reino Unido. pp. 25-49.
Tlaskalova-Hogenova H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Srůtková D, Zídek Z, Schwarzer M, Drastich P and Funda DP. 2011. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cellular and Molecular Immunology 8(2): 110-120. doi: 10.1038/cmi.2010.67
Turroni F, Ribbera A, Foroni E, van Sinderen D and Ventura M. 2008. Human gut microbiota and bifidobacteria: from composition to functionality. Antonie van Leeuwenhoek 94: 35-50. doi: 10.1007/s10482-008-9232-4.
Yang F, Hou C, Zeng X and Qiao S. 2015. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens 4: 34-45. doi: 10.3390/pathogens4010034
Yu I, Ju Ch-Ch, Lin J, Wu HL and Yen HT. 2004. Effects of probiotics and selenium combination on the immune and blood cholesterol concentration of pigs. Journal of Animal and Feed Science 13: 625-634.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Sumaya Tanzin Wahid, In Ho Kim. (2024). An evaluation of the effects of dietary mixed-species direct-fed microbial (probiotic) on growth performance, gas emissions, meat quality, nutrient digestibility, and fecal score in finishing pigs. Canadian Journal of Animal Science, 104(1), p.94. https://doi.org/10.1139/cjas-2023-0043.
2. Huan Wang, Byoung Duk Ha, In Ho Kim. (2021). Effects of probiotics complex supplementation in low nutrient density diet on growth performance, nutrient digestibility, faecal microbial, and faecal noxious gas emission in growing pigs. Italian Journal of Animal Science, 20(1), p.163. https://doi.org/10.1080/1828051X.2020.1801358.
3. Jaime A. Ángel-Isaza, Víctor Herrera Franco, Albeiro López-Herrera, Jaime E. Parra-Suescun. (2024). Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets. Veterinary Sciences, 11(8), p.332. https://doi.org/10.3390/vetsci11080332.
4. Chin‐Yen Tsai, Chia‐Chun Chi, Chun‐Hung Liu. (2019). The growth and apparent digestibility of white shrimp,Litopenaeus vannamei, are increased with the probiotic,Bacillus subtilis. Aquaculture Research, 50(5), p.1475. https://doi.org/10.1111/are.14022.
5. Samuel C.G. Jansseune, Aart Lammers, Jürgen van Baal, Fany Blanc, Marie-Hélène Pinard van der Laan, Fanny Calenge, Wouter H. Hendriks. (2024). Diet composition influences probiotic and postbiotic effects on broiler growth and physiology. Poultry Science, 103(6), p.103650. https://doi.org/10.1016/j.psj.2024.103650.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2016 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.