Mycorrhizal Dependency of Alcaparro (Senna pistaciifolia Kunth) at Three Concentrations of Soil Solution Phosphorus
Dependencia Micorrizal de la Alcaparra (Senna pistaciifolia Kunth) bajo Tres Concentraciones de Fósforo en la Solución del Suelo
DOI:
https://doi.org/10.15446/rfnam.v68n1.47831Keywords:
Glomus aggregatum, phosphate, plant nutrition, soil restoration. (en)Glomus aggregatum, fosfato, nutrición de plantas, recuperación de suelos. (es)
A greenhouse bioassay was carried out to determine the mycorrhizal dependency of alcaparro (Senna pistaciifolia Kunth). A randomized complete experimental design was employed, with six treatments in a factorial arrangement 3x2; the treatments consisted of three levels in soil solution phosphorus (P) (0.002, 0.02, and 0.2 mg L-1) combined with two levels of mycorrhizal inoculation either uninoculated or inoculated with Glomus agreggatum. The variables studied were leaf P content as a function of time, shoot dry matter, shoot P content, mycorrhizal colonization of roots, and the mycorrhizal dependency (MD). The results indicate that the leaf P content increased significantly with the mycorrhizal inoculation in alcaparro to 0.02 mg L-1 after the second sampling days, but not in the other soil P levels. Likewise, shoot dry weight increased significantly at 0.02 mg L-1. On the other hand, the total plant P content increase at all levels of soil available P. The mycorrhizal colonization in alcaparro roots was 11, 23, and 0% at 0.02, 0.002 and 0.2 mg of P L-1, respectively. The MD for alcaparro was 32%, which allow classify this specie as moderately dependent on the mycorrhizal association.
Mycorrhizal Dependency of Alcaparro (Senna pistaciifolia Kunth) at Three Concentrations of Soil Solution Phosphorus
Dependencia Micorrizal de la Alcaparra (Senna pistaciifolia Kunth) bajo Tres Concentraciones de Fósforo en la Solución del Suelo
Jorge Alberto Sierra Escobar1; Dagoberto Castro Restrepo2 and Nelson Walter Osorio Vega3
1 Assistant Professor. Universidad Católica de Oriente - Facultad de Ciencias Agropecuarias. Apartado Aéreo 008, Rionegro, Antioquia, Colombia. <jsierra@uco.edu.co>
2 Associate Professor. Universidad Católica de Oriente - Facultad de Ciencias Agropecuarias. Apartado Aéreo 008, Rionegro, Antioquia, Colombia. <dcastro@uco.edu.co>
3 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias - Escuela de Biociencias. A.A. 3840. Medellín, Colombia. <nwosorio@unal.edu.co>
Received: October 7, 2013; accepted: April 16, 2014
doi: https://doi.org/10.15446/rfnam.v68n1.47831
Abstract. A greenhouse bioassay was carried out to determine the mycorrhizal dependency of alcaparro (Senna pistaciifolia Kunth). A randomized complete experimental design was employed, with six treatments in a factorial arrangement 3x2; the treatments consisted of three levels in soil solution phosphorus (P) (0.002, 0.02, and 0.2 mg L-1) combined with two levels of mycorrhizal inoculation either uninoculated or inoculated with Glomus agreggatum. The variables studied were leaf P content as a function of time, shoot dry matter, shoot P content, mycorrhizal colonization of roots, and the mycorrhizal dependency (MD). The results indicate that the leaf P content increased significantly with the mycorrhizal inoculation in alcaparro to 0.02 mg L-1 after the second sampling days, but not in the other soil P levels. Likewise, shoot dry weight increased significantly at 0.02 mg L-1. On the other hand, the total plant P content increase at all levels of soil available P. The mycorrhizal colonization in alcaparro roots was 11, 23, and 0% at 0.02, 0.002 and 0.2 mg of P L-1, respectively. The MD for alcaparro was 32%, which allow classify this specie as moderately dependent on the mycorrhizal association.
Key words: Glomus aggregatum, phosphate, plant nutrition, soil restoration.
Resumen. Se realizó un bioensayo en invernadero para determinar la dependencia micorrizal de alcaparro (Senna pistaciifolia Kunth). Se utilizó un diseño experimental completamente randomizado. Los tratamientos se arreglaron en un factorial 3x2, estos consistieron en la combinación de tres niveles de P en la solución del suelo (0.002, 0.02 y 0.2 mg L-1) y 2 niveles de inoculación micorrizal (inoculado y no inoculado) con el hongo Glomus agreggatum. Se emplearon como variables respuesta el contenido de P foliar en función del tiempo; al momento de la cosecha, se determinaron la masa seca aérea, el P total en la parte aérea y la colonización micorrizal. Los resultados indican que el contenido de P foliar a 0.02 mg L-1 en el segundo muestreo aumentó significativamente con la inoculación micorrizal, pero no en los otros niveles de P. La masa seca aérea también creció significativamente a 0.02 mg L-1. De otro lado, el contenido de P total absorbido se incrementó significativamente en todos los niveles de P. La colonización micorrizal en las raíces del alcaparro fue de 11, 23 y 0 % para los niveles de 0.002, 0.02 y 0.2 mg L-1, respectivamente. La dependencia micorrizal fue del 32 %, por lo que esta especie se clasifica como moderadamente dependiente.
Palabras clave: Glomus aggregatum, fosfato, nutrición de plantas, recuperación de suelos.
One of the most successful strategies developed by plants to overcome the stress during the colonization of terrestrial ecosystems is the formation of symbiosis with arbuscular mycorrhizal fungi (Vosátka and Albrechtova, 2009). These fungi form mutualistic associations with the roots of plant species (Habte, 2006), which produces significant benefits for the plant host such as increase in water and nutrient uptake, improve in soil aggregate formation and stability, promote tolerance to plant pathogen, and favor positive interactions with other beneficial microorganisms (Mansfeld et al., 2002; Barea et al., 2002; Elsen et al., 2003; Johansson et al., 2004; Osorio and Habte, 2013). Although for some plant species the arbuscular mycorrhizal association is required for nutrient uptake and growth, the degree of mycorrhizal dependency (MD) varies among plant species. The MD was defined as the degree on which a plant species depends on the mycorrhizal association to reach a maximal growth and development (Plenchette et al., 1983).
In the last years in Colombia, several studies have been conducted to determine the MD of plant species such as Nageia rospigliosii (Diez et al., 2008), Solanum quitoense (Gonzalez and Osorio, 2008), Coffea arabiga (Jaramillo and Osorio 2009), Ocotea sp. (Sierra et al., 2009), Persea americana (Montoya and Osorio, 2009), Calophyllum brasiliense (Sierra et al., 2012), among others. These studies have confirmed the significance of mycorrhizal inoculation to promote plant nutrition and growth of agronomical and forestry plant species, particularly in soils with low phosphorus (P) availability, a major limiting factor for plant performance in the tropics (Osorio and Habte, 2013).
Among native forestry species in Colombia, alcaparro (Senna pistaciifolia Kunth) outstands because its use for restoration of degraded soils in Andean highlands, as a living fence, and as an ornamental plant (Alzate et al., 2012). Unfortunately, as it occurs with other plant species its growth and development is very slow, which impaired its use in reforestation and other uses, which has been associate to nutritional disorders particularly P deficiency (Haselwandter and Bowen, 1996; Rilling, 2004; Osorio et al., 2008; Diez 2011). Currently, there are not studies on nutritional requirements and the MD of alcaparro, which would allow land restorers and forestry managers to have a more effective use of this plant species. Then, the aim of this study was to determine its MD at three concentrations of soil solution phosphate.
MATERIALS AND METHODS
Site and soil preparation. This study was conducted in the greenhouse of the Universidad Católica de Oriente (6°9'15.2" N, 75°22'10.4" W, altitude 2.112 masl) (Rionegro, Antioquia - Colombia). The site had a mean air temperature of 17 °C, an annual rainfall of 1.700 mm, which correspond to ecological zone life of lower montane wet forest (Holdridge, 1967).
The soil in which plants grew was a sample of subsuperficial horizon (Bw, 30-50 cm) collected from and Andisol at the Forestry Station Piedras Blancas (6°15'38" N, 75°30'23" W, altitude 2.484 masl). This soil sample was used to reduce the influence of organic P on the level of soil solution P. The soil sample was air-dried, sieved at 4 mm, and then analyzed in the soil testing laboratory of the Universidad Nacional de Colombia at Medellín, Colombia. The results of these analyses were: sand 92%, silt 4%, clay 4%, soil texture sandy (Bouyucos), soil pH 5.7 (water, 1:1, V:V ), soil organic matter 11% (Walkley and Black), calcium, magnesium, and potassium: 1.7, 0.1, and 0.05 cmolc kg -1 (1 M ammonium acetate), respectively; phosphorus 1 mg kg-1 (Bray II); iron, manganese, copper, and zinc 64, 1, 1, and 1 mg kg-1 (Olsen - EDTA); boron non-detectable (<0.1 mg kg-1) (hot water); nitrate 3 mg kg-1 (0.025 M aluminum sulfate), and ammonium 18 mg kg-1 (1 M KCl). Details about the methods are available in Westerman (1990).
The soil pH was adjusted to 5.9 with the addition of CaCO3 (2 g kg-1) to improve calcium availability and optimize soil solution P. The lime requirement was determined by a lime incubation method (Uchida and Hue, 2000). Then, the soil was steamed at 80 °C for one hour. Soil fertilization was carried out with: urea 0.17 mg kg-1, CaSO4 2H2O 0.73 mg kg-1, MgSO4.7H2O 0.93 mg kg-1, and K2SO4 0.21 mg kg-1. Each planted bag received biweekly 25 mL of a P-free nutrient Hogland solution: (in mg L-1) N 50, K 132, Mg 106, S 204, Zn 10, Cu 5, B 0.8, and Mo 0.5. The soil moisture content was maintained between 39 and 47%, which corresponded to 50-60% of the maximal holding water capacity, respectively.
Host plant. The seeds of alcaparro were collected from mature and healthy trees planted in LLanogrande region at the Rionegro municipality (Antioquia, Colombia). These seeds were rinsed with distilled water and disinfected with 1% sodium hypochlorite solution and then transferred to germinate in sterile sand (Pedroza y Tupaz, 2008). Once germinated, each seedling was transplanted into plastic pots with sterile sand until reach 10 cm of height and finally transplanted in the plastic bags with the respective treatment.
Treatments. In addition to the soil fertilization described above, P was added as KH2PO4 in order to achieve three concentrations of soil solution P: 0.002, 0.02 and 0.2 mg L-1, according to Habte and Manjunath (1991) method for MD studies. To this purpose, an isotherm of P sorption was conducted with the Fox and Kamprath (1970) method (Figure 1). The amounts of KH2PO4 added were 0.0, 3.8, and 17.6 g kg-1, respectively. The sterile and fertilized soil was then transferred into plastic bags (diameter 18 cm and high 29.5 cm) over a greenhouse bench.
Before transplanting, the soil was inoculated (M) with 20 g kg-1 of a crude inoculum, which contained the mycorrhizal fungus Glomus agreggatum (Schenck and Smith modified by Koske) strain "UH-2002" suspended in a matrix soil: sand. The inoculum contained 8,500 infective mycorrhizal propagules per kg, which was determined by the most probable number technique (Porter, 1979). This fungus was originally supplied by M. Habte from the University of Hawaii (Honolulu, USA) and then multiplied in sorghum and kudzu roots following the method propossed by Habte and Osorio (2001). Its effectiveness to promote plant P uptake and growth has been extensively probed in a series of studies (Habte and Manjunath, 1991; Osorio and Habte, 2001; Jaramillo et al., 2004; Diez et al., 2008; Sierra et al., 2009, 2012) with native forestry species. The uninoculated soil received 20 g kg -1 of the autoclaved crude inoculum and 20 mL of filtrates from a 10% suspension made with the crude after removing mycorrhizal propagules fungal Whatman No. 1 filter paper.
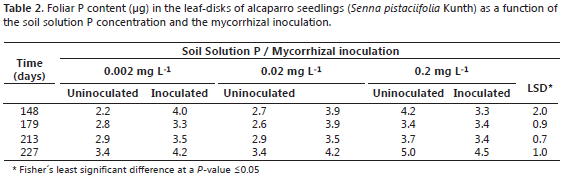
Measured variables. Leaf P content was monitored as a function of time in the youngest mature leaf following using the non-destructive sampling method developed by Aziz and Habte (1987). To this purpose, a leaf-disk (6 mm of diameter) was collected from this leaf, leaf samples were collected at 148, 179, 213, and 227 days after planting; samples were then ashed in a muffle furnace at 500 °C for three hours. Then, the ashes were dissolved in distilled water and the solution P concentration was measured by the blue-molybdate method (Murphy and Riley, 1962; Habte and Osorio, 2001). At harvesting (227 days after planting), the shoot dry weight (SDW) was determined after oven-dry the samples at 60 ºC for 72 h. Shoot P content (SPC) was measured as the product of shoot dry weight and the leaf P concentration at harvest. To determine root colonization by the fungus, 100 root fragment (1 cm) from fine roots were collected from each replicate and then were separately rinsed with water, cleared by immersion in 10% KOH solution for 24 h (Phillips and Hayman, 1970) and stained with fucshin acid (Kormanik et al., 1980) for 24 h. To quantify the presence of mycorrhizal structures we used the method of grid line intersection (Giovannetti and Mosse, 1980) in the 100 root fragments per replicate. The number of grid-line intercepts observed per sample was ca. 150.
The MD was evaluated as proposed by Plenchette et al. (1983) as the difference between SDW of inoculated and uninoculated plants, expressed as a percentage of SDW of inoculated plants. To this purpose data of SDW were used at each level of soil solution P level.
The mean value of MD obtained at the soil solution P of 0.02 mg L-1 was compared with the categories proposed by Habte and Manjunath (1991).
Experimental design and data analyses. The experimental design was completely randomized; treatments consisted of a factorial arrangement 3x2. This is, three levels of soil solution P: 0.002, 0.02, and 0.2 mg L-1 and two levels of mycorrhizal inoculation; each treatment had five replicates; this means that we had 30 experimental units. Data were subjected to analyses of variance and the Fisher's least significant difference (LSD) tests for mean separation at each level of soil solution P. Both tests were conducted with a significance level (P) 0.05 in the statistical software StatGraphics Plus version 4.0 (Statpoint, Inc., Herdon, Virginia, USA).
RESULTS AND DISCUSSION
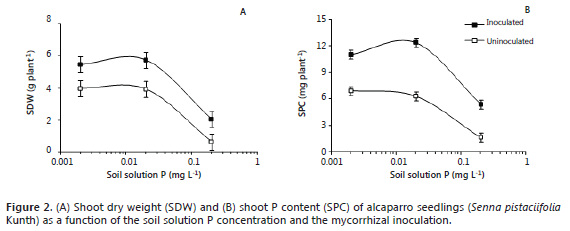
The mycorrhizal inoculation significantly increased the SDW of alcaparro seedlings, however, this effect varied with the level of soil soluble P (Figure 2A); for instance, at 0.002, 0.02, and 0.2 mg L-1 the magnitude of the increase of SDW respect to non-inoculated plants was 38, 47, and 218%, respectively. However, the value of SDW was significantly lower at the highest level of soil soluble P (0.2 mg L-1) in comparison with that at the 0.002 and 0.02 mg L-1. It is worth to mention that the soil pH was adjusted to 5.9, which favors the solubility of P ions (H2PO4-) (Osorio, 2012) and guaranteed the presence of soil soluble P in this soil.
The SPC was significantly increased by the mycorrhizal inoculation, but the magnitude of this effect also varied with the level of soluble P (Figure 2B). Thus, at 0.002, 0.02, and 0.2 mg L-1 the increase in SPC respect to the non-inoculated plants was 59, 96, and 225%, respectively. However, the value of SPC was lower at 0.2 mg L-1 than at the other soil P levels (Figure 2B).
Previous studies conducted with Leucaena species (Fabaceae) inoculated with G. agreggatum have produced contrasting results; for instance, L. leucocephala and L. diversifolia had high levels of mycorrizal colonization in their roots even at high soil P levels (0.2 mg L-1), while L. retusa and L. trichodes had lower levels of mycorrhizal colonization particularly at high soil P levels (Habte and Osorio, 2001), a tendency observed with alcaparro in the current study. Thus, the mycorrhizal colonization of alcaparro roots was 10.7, 22.9, and 0% at the soil soluble P levels of 0.002, 0.02, and 0.2 mg L-1, respectively (Table 1). No mycorrhizal structures were detected in uninoculated plants. Although the mean foliar P content was higher in inoculated plants than uninoculated plants, at least at 0.002 and 0.02 mg L-1, these differences were not significant, except at day 179 and at 0.02 mg L-1 (Table 2).
The results indicate that seedlings of alcaparro exhibited a MD of 32%, accordingly to Plenchete et al. (1983) formula. This plant species can be classified as moderately dependent on the mycorrhizal association (Habte and Manjunath, 1991). From the ecological point of view, alcaparro can be considered a pioneer plant species because it grows in deforested land or out of the forests. Other pioneer plants of the same genera Senna such S. macranthera and S. spectabilis have exhibited certain degree of mycorrhizal dependency, which favor their growth (Siqueira et al., 1998; Zangaro et al., 2005). However, Janos (1980a, 1980b) said that pioneer plants either form or not the mycorrhizal association.
The slight variation in shoot dry weight and shoot P content between the lowest and medium soil soluble P (0.002 and 0.02 mg L-1) suggest that alcapparro is well adapted to soil with low availability of P. At low levels of soil soluble P the fungus reduce the synthesis of sugars and phospholipids, which may increase the cell wall permeability and tranference to the cytoplasm (Genre y Bonfante, 1998). On the other hand, the poor plant performance at the high concentration of soluble P (0.2 mg L-1) seems to confirm this point. Also, it has been observed that at high levels of soil soluble P the root are more colonized by hyphae and less by arbuscules (the structures that exchange carbon from the plant to the fungus and nutrient, such P, from the fungus to the plant) (Osorio, 2012). High concentrations of soil available P can diminish the availability of some nutrients such as iron, manganese, copper, and zinc (Havlin et al., 2004). This may explain the decrease in plant growth and P uptake observed (Figure 2A, 2B). Similar results have been observed with other native species of the Andean mountains such as Laurel (Ocotea sp.) (Sierra et al., 2009), Barcino (Calophyllum brasiliense) (Sierra et al., 2012), Sweet granadilla (Passiflora ligularis) and Banana passion fruit (P. tripartita) (Osorio, 2012). For these reasons, we are reconsidering the use of the high level of soil solution P (0.2 mg L-1), at least for further studies of mycorrhizal dependency of forest species originated from high montane ecosystems. At these high levels of soil soluble P (0.2 mg L-1), these species had shown visual symptoms such as leaf and root necrosis likely associated to physiological disorders. Also, at this level the mycorrhizal colonization is impaired, which has been reported by some authors (Allen, 1996; Smith and Read, 1997; Habte and Osorio, 2001, Diez et al., 2008; Montoya and Osorio, 2009) and this current study.
From the practical point of view, these results suggest that the propagation of alcaparro seedlings at nursery should be accompanied by moderate application of P fertilizers (enough to reach a soil solution P concentration of 0.02 mg L-1) and by mycorrhizal inoculation. This clearly will promote the plant growth and the chance for a good establishment at the field will be high. On the other hand, since the arbuscular-mycorrhizal association is not specific for any plant-fungus combination (Walker y Trappe, 1993), it is possible to use any kind of fungus (e.g., G. aggregatum). However, it is likely to find better results with certain fungi in some plant species, which must be studied in the future not only for alcaparro but also for other plant species. In general terms and for practical reasons the use of an inoculum containing multi-species of arbuscular mycorrhizal fungi may be satisfactory in commercial nurseries.
CONCLUSIONS
The leaf P content increased significantly with the mycorrhizal inoculation in alcaparro to 0.02 mg L-1 after the second sampling days, but not in the other soil P levels. Likewise, shoot dry weight increased significantly at 0.02 mg L-1. On the other hand, the total plant P content increase at all levels of soil available P. The mycorrhizal colonization in alcaparro roots was 23, 11, and 0% at 0.02, 0.002 and 0.2 mg of P L-1, respectively. The MD for alcaparro was 32%, which allow classify this specie as moderately dependent on the mycorrhizal association.
ACKNOWLEDGEMENTS
This study was conducted by the financial support of the Universidad Catolica de Oriente, Direction of Research and Development and the Division of Plant Biotechnology. We thank all members of the Research Groups of Soil Microbiology of the Universidad Nacional de Colombia at Medellin for their academic support.
BIBLIOGRAPHY
Allen, M. 1996. The ecology of arbuscular mycorrhizas: a look back into the 20th century and a peek into the 21st. Mycological Research 100(7): 769-782.
Alzate, F., A. Idarraga, O. Diaz, and W. Rodríguez. 2012. Flora de los bosques montanos de Medellín. Universidad de Antioquia - Alcaldía de Medellín, Medellín. 552 p.
Aziz, T. and M. Habte. 1987. Determining vesicular-arbuscular micorrizal effectiveness by monitoring P status of leaf disk. Canadian Journal Microbiology 33: 1097-1101.
Barea, J.M., R. Azcón and C. Azcón. 2002. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leewenhoek 81(1-4): 343-351.
Diez, M.C. 2011. Conceptos importantes para la fertilización de plántulas de especies forestales. pp. 29-34. En: Diez, M.C., F.H. Moreno y Y. Sepúlveda (eds.). Fertilización de especies forestales de bosques andinos. Universidad Nacional de Colombia, Medellín. 75 p.
Diez, M.C., N.W. Osorio, and F.H. Moreno. 2008. Evaluation of mycorrhizal dependency of romeron pine (Nageia rospigliosii Pilger) under contrasting light conditions. Revista Facultad Nacional Agronomía Medellín 61(2): 4554-4563
Elsen, A., H. Baimey, R. Swennen and D. de Waele. 2003. Relative mycorrhizal dependency and mycorrhiza-nematode interaction in banana cultivars (Musa spp.) differing in nematode susceptibility. Plant and Soil 256:303-313.
Fox, R. and E. Kamprath. 1970. Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil Science Society of America Proceedings 34: 902-907.
Genre, A. and P. Bonfante. 1998. Actin versus tubulin configuration in arbuscule-containing cells from mycorrhizal tobacco roots. New Phytologist 140(4): 745-752.
Giovannetti, M. and B. Mosse. 1980. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist 84: 489-500.
González, O. and N.W. Osorio. 2008. Determinación de la dependencia micorrizal del lulo. Acta Biológica Colombiana 13(2): 163-174.
Habte, M. and A. Manjunath. 1991. Categories of vesicular-arbuscular mycorrhizal dependency of host species. Mycorrhiza 1:3-12.
Habte, M. and N.W. Osorio. 2001. Arbuscular Mycorrhizas: Producing and applying arbuscular mycorrhizal inoculum. University of Hawaii, Honolulu. 47 p.
Habte, M. 2006. The roles of arbuscular mycorrihizas in plant and soil health. pp. 129-147. In: N. Uphoff (ed.). Biological approaches to sustainable soil systems. CRC, Boca Raton, Florida. 764 p.
Haselwandter, K. and G.D. Bowen. 1996. Mycorrhizal relations in trees for agroforestry and land rehabilitation. Forest Ecology and Management 81(1-3): 1-17.
Havlin, J., J. Beaton, S.L.Tisdale and W. Nelson. 2004. Soil fertility and fertilizers. An introduction to nutrient management. Prentice Hall, Upper Saddle River, New Jersey. 478 p.
Holdridge, L.R. 1967. Life zone ecology. Tropical Science Center, San José, Costa Rica, 206 p.
Janos, D. 1980a. Mycorrhizae influence tropical succession. Biotropica 12(2): 54-64.
Janos, D. 1980b. Vesicular- arbuscular mycorrhizae affect lowland tropical rain forest plant growth. Ecology 61(1): 151-162.
Jaramillo, S.P., M. Silva and N.W. Osorio. 2004. Potencial simbiótico y efectividad de hongos micorrízico arbusculares de tres suelos sometidos a diferentes usos. Revista de la Facultad Nacional de Agronomía Medellín 57(1): 2203-2214.
Jaramillo, S.P. and N.W. Osorio. 2009. Mycorrhizal dependency of coffee seedling at different levels of soil solution phosphorus. Revista Suelos Ecuatoriales 39(1): 100-106.
Johansson, J.F., L.R. Paul and R.D. Finlay. 2004. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiology Ecology 48(1): 1-13.
Kormanik, P.P., W.C. Bryan and R.C. Schultz. 1980. Procedures and equipment for staining a large numbers of plant samples for endomycorrhizal assay. Canadian Journal Microbiology 26(4): 536-538.
Mansfeld, K., J. Larsen, and L. Bødker. 2002. Bacterial populations associated with mycelium of the arbuscular mycorrhizal fungus Glomus intrarradices. FEMS Microbiology Ecology 41(2):133-140.
Montoya, B. and N.W. Osorio. 2009. Mycorrhizal dependency of avocado at different levels of soil solution phosphorus. Suelos Ecuatoriales 39(2):143-147.
Murphy, J. and J.P. Riley. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31-36.
Osorio, N.W. 2012. Manejo de nutrientes en suelos del trópico. Universidad Nacional de Colombia, Medellín. 339 p.
Osorio, N.W. and M. Habte. 2001. Synergistic influence of an arbuscular micorrizal fungus and a P solubilizing fungus on growth and P uptake of Leucaena leucocephala in an oxisol. Arid Land Research and Management 15: 263-274.
Osorio, N.W. and M. Habte. 2013. Synergistic effect of a phosphate solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in an oxisol fertilized with rock phosphate. Botany 91(4): 274-281.
Osorio, W., M.C. Diez, J.A. Sierra, and L. Paternina. 2008. Consideraciones ecológicas sobre la asociación micorrizal en suelos de la región altoandina. pp. 181- 198. En: León, J.D. (ed.). Ecología de bosques altoandinos. Universidad Nacional de Colombia, Medellin. 260 p.
Pedroza, J.A y W.A. Tupaz. 2008. Micropropagación de Ilex kunthiana Triana and Planchon (Aquifoliaceae), una especie de gran importancia en programas de revegetalización. Revista Colombiana Biotecnológica 10(2): 78-84.
Phillips, J.M. and D.S. Hayman. 1970. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society 55(1): 158-161.
Plenchette, C., A. Fortin and V. Furlan. 1983. Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility. I. Mycorrhizal dependency under field conditions. Plant and Soil 70: 199-209.
Porter, W. 1979. The "Most Probable Number" method for ennumerating infective propagules of vesicular arbuscular micorrizal fungi in soil. Australian Journal of Soil Research 17: 515-519.
Rilling, M. 2004. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecology Letters 7(8):740-754.
Sierra, J.A., D. Castro y N.W. Osorio. 2009. Dependencia micorrizal de laurel (Ocotea sp.). Revista Colombia Forestal 12: 21-30.
Sierra, J.A., D. Castro and W. Osorio. 2012. Mycorrhizal dependence of barcino (Clusiaceae: Calophyllum brasiliense Cambers). Actualidades Biológicas 34(97): 199-206.
Siqueira, J.O., M.A. Carbone, N. Curi, S.C. Silva and A.C. Davide. 1998. Mycorrhizal colonization and mycotrophic growth of native woody species as related to successional groups in Southeastern Brazil. Forest Ecology and Management 107(1-3): 241-252
Smith, S. and D. Read. 1997. Mycorrhizal symbiosis. Academic Press, London. 330 p.
Walker, C. and J.M. Trappe. 1993. Names and epithets in the Glomales and Endogonales. Mycological Research 97(3): 339-344.
Uchida, R. and N.V. Hue. 2000. Plant nutrient management in Hawaii's soils, approaches for tropical and subtropical agriculture. pp. 101-111. In: Silva, J.A. and R. Uchida (eds.). College of Tropical Agriculture and Human Resources. University of Hawaii at Manoa. 158 p.
Vosatka, M. and J. Albrechtova. 2009. Benefits of arbuscular mycorrhizal fungi to sustainable crop production. pp. 205-225. In: Khan, M.S. (ed.). Microbial strategies for crop improvement. Springer-Verlag, Berlin. 358 p.
Westerman, R.L. 1990. Soil Testing and Plant Analysis, Soil Science Society of America, ASA, Madison, Wisconsin. 870 p.
Zangaro, W., F.R. Nishidate, F.R. Spago-Camargo, G. Gorete-Romagnoli and J. Vandressen. 2005. Relationships among arbuscular mycorrhizas, root morphology and seedling growth of tropical native woody species in southern Brazil. Journal of Tropical Ecology 21(5): 529-540.
References
Allen, M. 1996. The ecology of arbuscular mycorrhizas: a look back into the 20th century and a peek into the 21st. Mycological Research 100(7): 769-782.
Alzate, F., A. Idarraga, O. Diaz, and W. Rodríguez. 2012. Flora de los bosques montanos de Medellín. Universidad de Antioquia - Alcaldía de Medellín, Medellín. 552 p.
Aziz, T. and M. Habte. 1987. Determining vesicular-arbuscular micorrizal effectiveness by monitoring P status of leaf disk. Canadian Journal Microbiology 33: 1097-1101.
Barea, J.M., R. Azcón and C. Azcón. 2002. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leewenhoek 81(1-4): 343-351.
Diez, M.C. 2011. Conceptos importantes para la fertilización de plántulas de especies forestales. pp. 29-34. En: Diez, M.C., F.H. Moreno y Y. Sepúlveda (eds.). Fertilización de especies forestales de bosques andinos. Universidad Nacional de Colombia, Medellín. 75 p.
Diez, M.C., N.W. Osorio, and F.H. Moreno. 2008. Evaluation of mycorrhizal dependency of romeron pine (Nageia rospigliosii Pilger) under contrasting light conditions. Revista Facultad Nacional Agronomía Medellín 61(2): 4554-4563
Elsen, A., H. Baimey, R. Swennen and D. de Waele. 2003. Relative mycorrhizal dependency and mycorrhiza-nematode interaction in banana cultivars (Musa spp.) differing in nematode susceptibility. Plant and Soil 256:303-313.
Fox, R. and E. Kamprath. 1970. Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil Science Society of America Proceedings 34: 902-907.
Genre, A. and P. Bonfante. 1998. Actin versus tubulin configuration in arbuscule-containing cells from mycorrhizal tobacco roots. New Phytologist 140(4): 745-752.
Giovannetti, M. and B. Mosse. 1980. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist 84: 489-500.
González, O. and N.W. Osorio. 2008. Determinación de la dependencia micorrizal del lulo. Acta Biológica Colombiana 13(2): 163-174.
Habte, M. and A. Manjunath. 1991. Categories of vesicular-arbuscular mycorrhizal dependency of host species. Mycorrhiza 1:3-12.
Habte, M. and N.W. Osorio. 2001. Arbuscular Mycorrhizas: Producing and applying arbuscular mycorrhizal inoculum. University of Hawaii, Honolulu. 47 p.
Habte, M. 2006. The roles of arbuscular mycorrihizas in plant and soil health. pp. 129-147. In: N. Uphoff (ed.). Biological approaches to sustainable soil systems. CRC, Boca Raton, Florida. 764 p.
Haselwandter, K. and G.D. Bowen. 1996. Mycorrhizal relations in trees for agroforestry and land rehabilitation. Forest Ecology and Management 81(1-3): 1-17.
Havlin, J., J. Beaton, S.L.Tisdale and W. Nelson. 2004. Soil fertility and fertilizers. An introduction to nutrient management. Prentice Hall, Upper Saddle River, New Jersey. 478 p.
Holdridge, L.R. 1967. Life zone ecology. Tropical Science Center, San José, Costa Rica, 206 p.
Janos, D. 1980a. Mycorrhizae influence tropical succession. Biotropica 12(2): 54-64.
Janos, D. 1980b. Vesicular- arbuscular mycorrhizae affect lowland tropical rain forest plant growth. Ecology 61(1): 151-162.
Jaramillo, S.P., M. Silva and N.W. Osorio. 2004. Potencial simbiótico y efectividad de hongos micorrízico arbusculares de tres suelos sometidos a diferentes usos. Revista de la Facultad Nacional de Agronomía Medellín 57(1): 2203-2214.
Jaramillo, S.P. and N.W. Osorio. 2009. Mycorrhizal dependency of coffee seedling at different levels of soil solution phosphorus. Revista Suelos Ecuatoriales 39(1): 100-106.
Johansson, J.F., L.R. Paul and R.D. Finlay. 2004. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiology Ecology 48(1): 1-13.
Kormanik, P.P., W.C. Bryan and R.C. Schultz. 1980. Procedures and equipment for staining a large numbers of plant samples for endomycorrhizal assay. Canadian Journal Microbiology 26(4): 536-538.
Mansfeld, K., J. Larsen, and L. Bødker. 2002. Bacterial populations associated with mycelium of the arbuscular mycorrhizal fungus Glomus intrarradices. FEMS Microbiology Ecology 41(2):133-140.
Montoya, B. and N.W. Osorio. 2009. Mycorrhizal dependency of avocado at different levels of soil solution phosphorus. Suelos Ecuatoriales 39(2):143-147.
Murphy, J. and J.P. Riley. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31-36.
Osorio, N.W. 2012. Manejo de nutrientes en suelos del trópico. Universidad Nacional de Colombia, Medellín. 339 p.
Osorio, N.W. and M. Habte. 2001. Synergistic influence of an arbuscular micorrizal fungus and a P solubilizing fungus on growth and P uptake of Leucaena leucocephala in an oxisol. Arid Land Research and Management 15: 263-274.
Osorio, N.W. and M. Habte. 2013. Synergistic effect of a phosphate solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in an oxisol fertilized with rock phosphate. Botany 91(4): 274-281.
Osorio, W., M.C. Diez, J.A. Sierra, and L. Paternina. 2008. Consideraciones ecológicas sobre la asociación micorrizal en suelos de la región altoandina. pp. 181- 198. En: León, J.D. (ed.). Ecología de bosques altoandinos. Universidad Nacional de Colombia, Medellin. 260 p.
Pedroza, J.A y W.A. Tupaz. 2008. Micropropagación de Ilex kunthiana Triana and Planchon (Aquifoliaceae), una especie de gran importancia en programas de revegetalización. Revista Colombiana Biotecnológica 10(2): 78-84.
Phillips, J.M. and D.S. Hayman. 1970. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society 55(1): 158-161.
Plenchette, C., A. Fortin and V. Furlan. 1983. Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility. I. Mycorrhizal dependency under field conditions. Plant and Soil 70: 199-209.
Porter, W. 1979. The "Most Probable Number" method for ennumerating infective propagules of vesicular arbuscular micorrizal fungi in soil. Australian Journal of Soil Research 17: 515-519.
Rilling, M. 2004. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecology Letters 7(8):740-754.
Sierra, J.A., D. Castro y N.W. Osorio. 2009. Dependencia micorrizal de laurel (Ocotea sp.). Revista Colombia Forestal 12: 21-30.
Sierra, J.A., D. Castro and W. Osorio. 2012. Mycorrhizal dependence of barcino (Clusiaceae: Calophyllum brasiliense Cambers). Actualidades Biológicas 34(97): 199-206.
Siqueira, J.O., M.A. Carbone, N. Curi, S.C. Silva and A.C. Davide. 1998. Mycorrhizal colonization and mycotrophic growth of native woody species as related to successional groups in Southeastern Brazil. Forest Ecology and Management 107(1-3): 241-252
Smith, S. and D. Read. 1997. Mycorrhizal symbiosis. Academic Press, London. 330 p.
Walker, C. and J.M. Trappe. 1993. Names and epithets in the Glomales and Endogonales. Mycological Research 97(3): 339-344.
Uchida, R. and N.V. Hue. 2000. Plant nutrient management in Hawaii's soils, approaches for tropical and subtropical agriculture. pp. 101-111. In: Silva, J.A. and R. Uchida (eds.). College of Tropical Agriculture and Human Resources. University of Hawaii at Manoa. 158 p.
Vosatka, M. and J. Albrechtova. 2009. Benefits of arbuscular mycorrhizal fungi to sustainable crop production. pp. 205-225. In: Khan, M.S. (ed.). Microbial strategies for crop improvement. Springer-Verlag, Berlin. 358 p.
Westerman, R.L. 1990. Soil Testing and Plant Analysis, Soil Science Society of America, ASA, Madison, Wisconsin. 870 p.
Zangaro, W., F.R. Nishidate, F.R. Spago-Camargo, G. Gorete-Romagnoli and J. Vandressen. 2005. Relationships among arbuscular mycorrhizas, root morphology and seedling growth of tropical native woody species in southern Brazil. Journal of Tropical Ecology 21(5): 529-540.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Nagaraj Kandhasamy, Koshila Ravi Ravichandran, Muthukumar Thangavelu. (2020). Interactive Influence of Soil and Plant Genotypes on Mycorrhizal Dependency in Finger Millet. Journal of Soil Science and Plant Nutrition, 20(3), p.1287. https://doi.org/10.1007/s42729-020-00212-2.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2015 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.