ABUNDANCE AND ISOTOPIC COMPOSITION OF PLANKTONIC MICROCRUSTACEANS IN A CENTRAL AMAZON FLOODPLAIN LAKE: IMPLICATIONS FOR THE TROPHIC DYNAMICS OF THE PLANKTON COMMUNITY
DOI:
https://doi.org/10.15446/caldasia.v38n1.57835Palabras clave:
Amazon, planktonic community, stable isotopes. (es)During the hydrological year from December 2007 to November 2008, monthly samplings in the pelagic, littoral and macrophytes zones were conducted in the Lago Catalão, a floodplain lake receiving a mixture of water from Negro and Solimões Rivers, in front of Manaus city. Taxonomic composition and their relative abundance of the planktonic microcrustaceans community was studied. Natural abundances of carbon (C) and nitrogen (N) stable isotopes were measured to indicate energy sources. Cladocerans were the most abundant, with a relative abundance of 60%, followed by the calanoid and cyclopoid copepods with relative abundances of 29% and 11%, respectively. Diaphanosoma spp. was the dominant cladoceran group during all the sampling periods. Cladocerans were also represented by Moina spp., Ceriodaphnia spp. and Daphnia gessneri. Three genera of calanoid copepods were found: Notodiaptomus spp, Rhacodiaptomus spp., and Argyrodiaptomus spp. The genus Mesocyclops spp. was identified among the cyclopoid copepods. Zooplankton δ13C values indicated that the aquatic macrophyte zone was distinct, with a mean of -27.31‰, which was more enriched than zooplankton in the pelagic and littoral zones, where they had mean δ13C values of -33.11 and -34.66‰, respectively. Overall, analysis of stable isotopes showed that regardless of the pathways, the initial source of carbon for the zooplankton was phytoplankton, with a minimal participation of heterotrophic bacteria.
PEDRO CARABALLO
BRUCE FORSBERG
ROSSEVAL LEITE
Universidad de Sucre, Colombia, grupo de Biodiversidad Tropical. Autor para correspondencia: pedro.caraballo@unisucre.edu.co
Instituto Nacional de Pesquisas da Amazônia INPA, CEP 69067-375, Manaus, AM, Brazil. brforsberg@gmail.com, rosseval@gmail.com
ABSTRACT
During the hydrological year from December 2007 to November 2008, monthly samplings in the pelagic, littoral and macrophytes zones were conducted in the Lago Catalão, a floodplain lake receiving a mixture of water from Negro and Solimões Rivers, in front of Manaus city. Taxonomic composition and their relative abundance of the planktonic microcrustaceans community was studied. Natural abundances of carbon (C) and nitrogen (N) stable isotopes were measured to indicate energy sources. Cladocerans were the most abundant, with a relative abundance of 60%, followed by the calanoid and cyclopoid copepods with relative abundances of 29% and 11%, respectively. Diaphanosoma spp. was the dominant cladoceran group during all the sampling periods. Cladocerans were also represented by Moina spp., Ceriodaphnia spp. and Daphnia gessneri. Three genera of calanoid copepods were found: Notodiaptomus spp, Rhacodiaptomus spp., and Argyrodiaptomusspp. The genus Mesocyclops spp. was identified among the cyclopoid copepods. Zooplankton δ13C values indicated that the aquatic macrophyte zone was distinct, with a mean of -27.31, which was more enriched than zooplankton in the pelagic and littoral zones, where they had mean δ13C values of -33.11 and -34.66, respectively. Overall, analysis of stable isotopes showed that regardless of the pathways, the initial source of carbon for the zooplankton was phytoplankton, with a minimal participation of heterotrophic bacteria.
Key words. Amazon, planktonic community, stable isotopes.
RESUMEN
Durante el año hidrológico de diciembre 2007 a noviembre de 2008, se realizaron muestreos mensuales del zooplancton en las regiones pelágica, litoral y de macrófitas acuáticas, en el lago Catalão, un lago de aguas mixtas en la región de confluencia de los ríos Negro y Solimões, frente a la ciudad de Manaus. La comunidad de los microcrustáceos planctónicos se estudió en términos de su abundancia relativa, así como la abundancia natural de los isótopos estables de carbono y nitrógeno para definir las fuentes de energía. Los cladóceros fueron más abundantes en todas las regiones estudiadas, presentando una media de 60%, seguido por los copépodos calanoideos con 29% y los ciclopoideos con 11%. Los cladóceros estuvieron representados por Moina spp., Ceriodaphniaspp., Daphnia gessneri y como grupo dominante, Diaphanosoma spp. durante todo el período de estudio. Sólo los copépodos adultos fueron contados e entre estos, tres géneros de calanoideos fueron identificados, Notodiaptomus spp., Rhacodiaptomusspp. y Argyrodiaptomus spp., sin embargo la abundancia individual de los géneros no fue evaluada. Entre los ciclopoideos, fue identificado el género Mesocyclops spp. La abundancia de δ13C en las tres regiones estudiadas presenta a la región de macrófitas acuáticas como un área diferenciada en términos de la alimentación del zooplancton, con una media de -27,31, que es más enriquecida que las regiones litoral y pelágica, con valores de -33,11 e -34,66 respectivamente. El uso del análisis de isótopos estables de carbono y nitrógeno permitió definir que, independiente de las vías por las cuales el carbono este circulando, la fuente inicial de carbono para el zooplancton es principalmente el fitoplancton, con una mínima participación de las bacterias heterotróficas.
Palabras clave. Amazonia, comunidad planctónica, análisis de isótopos estables.
Recibido: 09/07/2013
Aceptado: 23/04/2016
INTRODUCTION
The central role of the zooplankton in the carbon flow to upper trophic levels in lakes is well established, but the origin of the carbon (energy) that supports zooplankton is less well understood. Studies carried out in lakes of temperate zones have shown that zooplankton use carbon from both phytoplankton and terrestrial sources as energy sources (Carpenter et al. 2005), which would include aquatic macrophytes and flooded forests in the case of many tropical regions. The major uncertainty involves the role of detrital organic carbon, which may originate from one or more of the autotrophic sources and may be obtained either directly from the consumption of particulate organic carbon or indirectly via consumption of bacteria or fungi which are in turn dependent on detrital organic matter (Porter 1996). This uncertainty is due in part to the diversity of potential zooplankton dietary sources including phytoplankton, detritus, bacteria, and protozoans, the relative importance of which depends on the community species composition and environmental conditions including, in the case of floodplain lakes, the seasonal flood pulse.
In lakes, zooplankton communities are ecologically diverse and temporally and spatially dynamic, and specifically in floodplain lakes, the composition and abundance of zooplankton communities change frequently under the influence of abiotic and biotic factors (Torres-Bejarano et al. 2013). In floodplain lakes, zooplankton populations has been shown to be influenced by predation by fishes and by invertebrates such Chaoborus (Twombly & Lewis 1989). Evidence of top-down control of zooplankton comes from the analysis of the size distributions of zooplankton in lakes of Central Amazon presented by Trevisan & Forsberg (2007), who found predominantly small zooplankton in all lakes they studied, which indicates high predation pressure. Nevertheless, the same authors found a significant correlation between the abundance of zooplankton and phytoplankton indicating that bottom-up effects of resource limitation were also important. A study of the zooplankton population dynamics in a floodplain lake in the Orinoco River in Venezuela showed high birth rates throughout the hydrological year, which were indicative of high predation rates (Twombly & Lewis 1987, 1989). Based on in situ experiments in a lake in Bolivia, Rejas et al. (2005) also found a constant birth rate, however, their results suggest an alternating bottom-up and top-down control of the zooplankton, especially those of greater size. It is likely that the abundance of the zooplankton in all of these systems is controlled simultaneously by the top-down (predation) and bottom-up effects, as shown for phytoplankton in several lakes of the Northern hemisphere (Mazumder 1994). However, the top-down effect apparently predominates in the majority of the floodplain lakes of Central Amazon that have been studied.
Population dynamics analysis conducted by different researchers in floodplain lakes have shown a higher zooplankton density during low water period (Brandorff & Andrade 1978, Lindholm & Hessen 2007), but this does not necessarily imply greater abundance because these lakes vary seasonally in volume. Considering the enormous fluctuations of the water level in Amazon lakes, which for instance during 2007, was 18 m in the Rio Negro, the zooplankton populations may increase in number of individuals as they decrease in density (Twombly & Lewis 1987). This was observed in the Lago Calado by Caraballo (1992), who used the estimates of zooplankton density together with the data of Lesack (1988) to calculate the volume of that lake, and express the results in terms of zooplankton abundance, demonstrating the highest zooplankton abundance during the flooding, even though densities were lower.
The relation between zooplankton population dynamics and hydrological phases is not equal for all components of the zooplankton community, which includes four large groups: protists, rotifers, cladocerans and copepods. In general, the species richness is greatest among the protists and smallest among the copepods. In fact, the trophic position of the zooplankton varies with the taxa, the hydrological phase, and size of the organisms. For example, Trevisan & Forsberg (2007) compared abundance and zooplankton size distributions in floodplain lakes associated with the Rio Solimões, Rio Negro and a floodplain lake receiving water from both of those rivers (Lago Catalão). They found a mean density of 19.8 ind./L from which only 17.8% were large, corresponding to mesozooplankton following Sieburth et al. (1978) classification, which comprises organisms of sizes ranging 200-2000 µm, in which the cladocerans and copepods dominate. Contrasting abundances and sizes were found in lakes of Rio Solimões (47.4 ind./L and 14.5% large) and Rio Negro (3.7 ind./L and 4.9% large). In Lago Catalão, they found a mean of 185 ind./L total, of which 25% were large.
Zooplankton community responses to the seasonal changes in the floodplain lakes have been documented in terms of composition and abundance (Brandorff & Andrade 1978, Lindholm & Hessen 2007), but without a clear explanation of how such zooplankton communities respond to seasonal variations in the spatial distribution of their energy resources in the lakes. Looking for an explanation, Yoshioka et al. (1994) used stable isotopic analysis of the zooplankton and its autotrophic sources of carbon in a Japanese lake. Since the C isotopic ratio (expressed as δ13C) of a consumer reflects the isotopic composition of its diet (Post 2002) and the nitrogen isotopic ratio (δ15N) indicates the trophic position of an organism (Vander Zanden & Rasmussen 1996), the simultaneous analysis of C and N isotope ratios can reveal how the carbon is transferred from primary energy sources to upper trophic levels (Smyntek et al. 2007).
This study analysed the zooplankton community of Lago Catalão, an Amazon floodplain lake, using stable C and N isotopes to reveal the relative importance of different autotrophic sources of carbon to the zooplankton.
MATERIAL AND METHODS
Area of study. Lago Catalão is located on the floodplain near the confluence of Solimões and Negro Rivers (3°10'04''S and 59°54'45''W), 3 km from Manaus (Fig. 1). The lake lies on a peninsula that has a series of interconnected water bodies that can become a continuous water surface during inundation, but become isolated from each other and may dry completely at low water levels, normally in Oct-Nov (Brito 2006). Normally, Lago Catalão is connected to the Rio Negro directly by a channel and it is seasonally connected to Rio Solimões through a wide area during flooding. Thus, it is a lake with alternating dominance of Rio Negro in the low-water period, and the Rio Solimões at the end of the high water period.
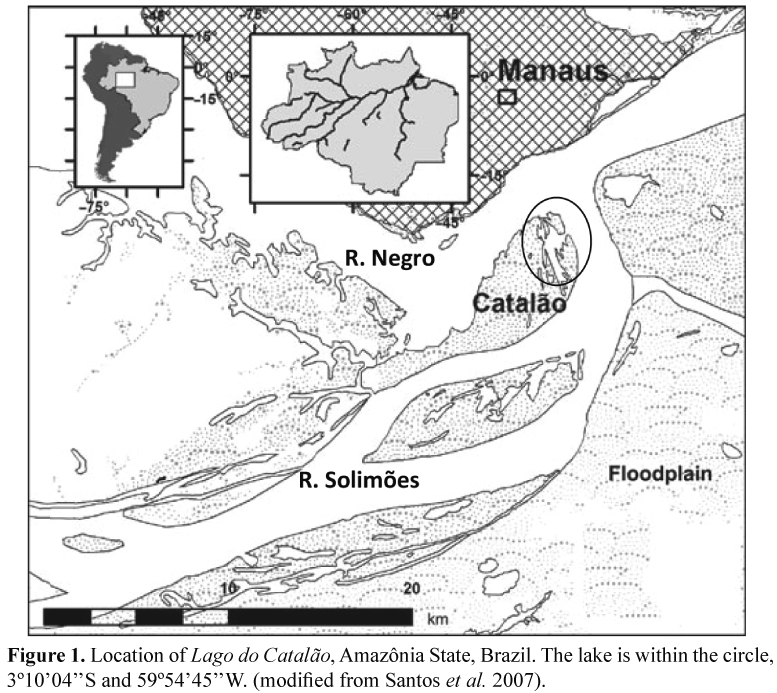
The lake presents extreme environmental variation between the low water and flood phases. Brito (2006) and Almeida & Melo (2009) observed values of conductivity up to 400 µS/cm in the low-water phase, which decreased with the entrance of water from the Rio Negro down to 30 µS/cm, and increased again to 70 µS/cm, after the entrance of water from the Rio Solimões, becoming similar n chemistry to that river during flooding. The abundance of phytoplankton was strongly related to conductivity, presenting mean values of 9.0 µg/L of total chlorophyll and primary productivity of 29.1 ± 17.9 mg C/m3/h during the flooding (Brito 2006), being dominated by the cyanophyceae, specially Synechocystis aquatilis, Synechococcus elongates, and Planktothrix isothrix (Almeida & Melo 2009).
The hydrological phases of isolation, filling, flooding, and falling waters are well-defined in the lake, as they are the direct result of the predictable flooding regime of both rivers. Figure 2 presents the dynamic of the water level at the Port of Manaus, which corresponds to the water level of the lake. On November 22nd 2007, when the level of the Rio Negro at Manaus was 18.9 m, the lakes started to receive water from this river, and when the level reached 25.6 m on April 27th 2007, the lake started to receive water from the Rio Solimõesthrough its northern channel. The flooding phase occurred between June and July, followed by the falling water phase between August and September, and the isolation phase between October and November, when the lake did not receive water from any of the adjacent rivers, and became much reduced in area.
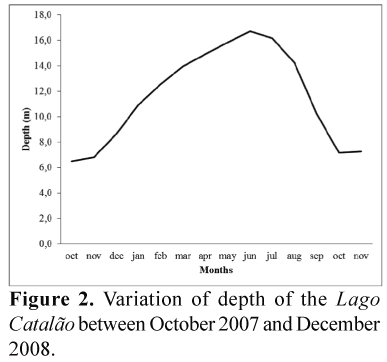
Low transparency (0.3 m Secchi depth) and high conductivity characterize the isolation phase in the lake, when it resembled other kinds of isolated lakes. At the end of November, the inflow began with the input of water from the Rio Negro, diluting the water of the lake, increasing the transparency and decreasing the conductivity. The minimal conductivity was reached at the end of April (24 µS/cm); at the same time, the transparency reached its maximum level (1-1.2 m; Brito 2006). Such levels remained stable until the beginning of May, when the lake reached 12 metres of depth and began to receive input of water from the Rio Solimões, promoting a homogenization of the lake due to the mixture of the waters. In June-July, the flooding phase occurred, with smaller fluctuations of the water level and maximum transparency of the water (1.3 m). By the end of this phase, the floating macrophytes had disappeared from the lake.
Sampling. Monthly samplings were conducted from December 2007 to November 2008 in the open water, littoral, and macrophyte zones of the lake. In all environments, independent of the sampling technique, 30 L of water were filtered through a 65 µm sieve; the organisms retained by the sieve were preserved in 4% formalin. PVC tubes of 10 cm diameter and 6 m length were used for sampling the pelagic and coastal regions. The depth was always three times deeper than Secchi depth. The tube had a device that allows the entrance of water when it is lowered into the water column, and hinders the entrance of water when it is pulled to the surface, which results in a vertically integrated sample of the entire photic zone. At the shallower littoral zone, the tube was lowered diagonally until it approached the bottom, without touching the sediment. In the sampling within the floating meadows, a 10 L bucket was filled and the sample filtered through 200 µm mesh to remove residuals of roots and other debris before the final collection of zooplankton on a 65 µm sieve.
The relative abundance was calculated based on counting, under a stereoscopic microscope, of 50% of the volume of the samples. Fish and insect larvae, especially from the littoral and aquatic macrophyte environments, were not counted, but removed from the samples for individual analysis of stable isotopes.
Zooplankton samples for stable isotope analyses were collected using a conical net of 65 µm mesh, 50 cm in diameter, and 1.1 m long, taking horizontal and vertical tows in the open water zone and horizontal tows in the littoral zone, and collecting water with a bucket below the aquatic macrophytes. In all cases, sampling was carried out until a visible amount of organisms was captured. These organisms were kept in distilled water up to 3 hours, changing the water every hour, to clear their intestinal contents (Smyntek et al. 2007).
Preparation of the samples for stable isotope analysis (SIA). Samples were filtered onto GF/C glass-fiber filters that had been pre-combusted for one hour at 450ºC and dried at 60ºC for 12 hours. Depending on the amount of the sample, the filter or the total zooplankton mass was kept in desiccator until the shipping for isotope ratio mass spectrometric analysis (IRMS/EA) at the Environmental Stable Isotope Centre of the Bioscience Institute/ Unesp/ Botucatu, Brasil. The samples, which were free from impurities and contaminants, were dried in a forced-air oven at 50ºC and then pulverized to ≤250 µm in a liquid nitrogen-based cryogenic mill (-196ºC). To avoid cross-contamination, a mass of 0.5 mg is enough for 13C/12C ratio isotopic analysis with two replicates, and an error below 0.2.
In general, isotopic analyses were conducted on samples of total zooplankton mass. In cases of large abundance of a specific group, separate samples were prepared as well. The isotopic analysis of the potential feeding sources of the zooplankton included the C3 and C4 aquatic macrophytes, phytoplankton according to the methodology proposed by Forsberg et al. (1993), and detritus and heterotrophic bacteria according to Coffin et al. (1989), modified by Caraballo et al. (2012). As complementary information, monthly assessments of depth and transparency were carried in the open water zone of the lake.
Variability of d δ13C and δ15N values is presented as an average values ± standard deviation (sd) and analyzed using a non-parametric Kruskal-Wallis test, following of a Kolmogorov-Smirnov test. The significance level was p<0,05.
RESULTS
Lago Catalão is a "mixed water" floodplain lake, influenced alternately by two of the largest rivers in the world. In the beginning of the riverine inflow in November 2007, the lake received black water from the Rio Negro, lasting through July. However, at the end of April, when the lake had reached a depth of 12 m, the Rio Solimões began to bring white water through the northern part of the lake, in a process that can block the intake of the water from Rio Negro, whose entry channel became dammed by the lake. During this flood pulse period, besides depth, strong variations in transparency were observed (Fig. 2). The highest levels of transparency were found in the months of January and February, when the lake received water exclusively from the Rio Negro. The level of transparency decreased with the inflow of water of Rio Solimõesin April, then increased later in the high water period (Caraballo et al. 2014).
After the second sampling, the low number of rotifers in the samples suggested that the sieves were not retaining this group, and therefore they were not counted. This observation was supported by the low biomass and the small contribution of rotifers to samples for stable isotope analysis. The cladocerans were represented by Moina spp., Ceriodaphnia spp., Daphnia gessneri, and particularly by the dominant genus Diaphanosoma spp. during the entire sampling period. Only the adult copepods were counted; among them, three genera of calanoids were found Notodiaptomusspp., Rhacodiaptomus spp.and Argyrodiaptomusspp. however, the individual abundance of the genera was not assessed. Among the cyclopoids, we identified the genus Mesocyclops. Other organisms such Chaoborus, fish larvae and insects of the family Corixidae appeared occasionally in the samples while we disregarded them from the zooplankton counting, they were included in the isotopic analysis. In the case of the larvae of Chaoborus, night samples were performed exclusively for its sampling.
The cladocerans were the most abundant organisms in all of the sampled zones, presenting a mean of 60%, followed by the calanoid with 29% and cyclopoid with 11%. Such zooplankton abundance was higher in the open water zone and lower in the aquatic macrophyte zone (Fig. 3). Diaphanosoma was the only genus that was present in all months of sampling, while Daphnia gessneri only appeared at the end of the flow and flood, in both cases being associated with low levels of turbidity in the water.

Comparing the three zones of the lake, the largest dominance of the cladocerans was found in the open water zone (Fig. 4B), and the smallest found at the macrophyte zone, where cladoceran abundance was still >50%. In the littoral zone, calanoid copepods dominated temporarily at the beginning of the inflow (Fig. 4A), together with a strong presence of larval fish and insects of the family Corixidae. At the aquatic macrophyte zone, the proportion of cyclopoid copepods became larger during the falling water phase (August and September), when the plants were in senescence (Fig. 4C). In fact, during October and November (isolation phase), there were not floating macrophyte in the Lago Catalão.

During this study, the values of δ13C in the zooplankton were between -21.79 (at the aquatic macrophyte zone in May) and -38.48 (littoral zone in September). The largest range in zooplankton δ13C (11.43) was observed in the littoral zone. The open water zone was more stable, showing a range of 8.01 in zooplankton δ13C. The aquatic macrophyte zone was more dynamic (p < 0.05) when compared to the other two zones, showing a mean zooplankton δ13C of -27.31, which is more 13C-enriched than the values from the littoral and open-water zones, which averaged -33.11 and -34.66, respectively (Fig. 5).

In the other hand, zooplankton δ15N showed more uniform values among the three zones, varying from 9.73 (December, in the open-water zone) to 4.71 (March, in the aquatic macrophyte zone). The littoral zone had the lowest variability in zooplankton δ15N (range, 2.88), whereas the aquatic macrophyte zone showed the highest variability (4.17). A seasonal oscillation of the zooplankton δ15N values was observed in all three zones, but a strong and general decrease was evident in the period between December and April, which corresponds to flooding with water from the Rio Negro. An increase in δ15N (until June) was evident with the input of the water from the Rio Solimões at the end of April, decreasing again in August and September (Fig. 6).

Values of zooplankton δ13C identified the aquatic macrophytes as a differentiated zone regarding zooplankton feeding behaviour, with a mean of -27.31, much more enriched than the zooplankton of the littoral and pelagic zones, with had values of -33.11 and -34.66, respectively (Fig. 7). Zooplankton in the littoral zone was enriched in 13C during the flood phase, and later presented minimal values during falling water and isolation phases. Such a tendency, even less pronounced, was also observed for the open-water zone, which presented the lowest variability in zooplankton δ13C. Highest variability was found in the open-water and aquatic macrophyte zones during the flooding phase. The values of zooplankton δ13C were more negative in the open-water and littoral zones during the falling water and isolation phases.
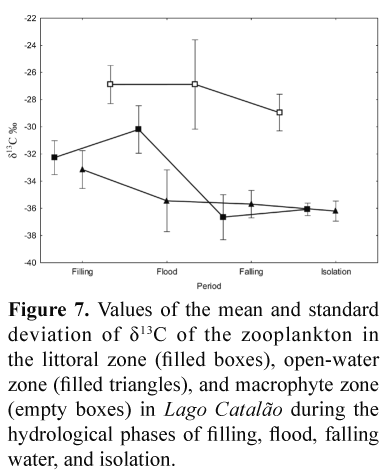
Values of zooplankton δ15N showed high variability, being relevant the case of the aquatic macrophyte zone during the flow, with values ranging from 4.71-8.88, suggesting a range across two trophic levels if the basal δ15N source were the same (Fig. 8). The highest values of δ15N were found in the open-water zone during the flooding phase and the lowest in the aquatic macrophyte zone during the falling water phase.

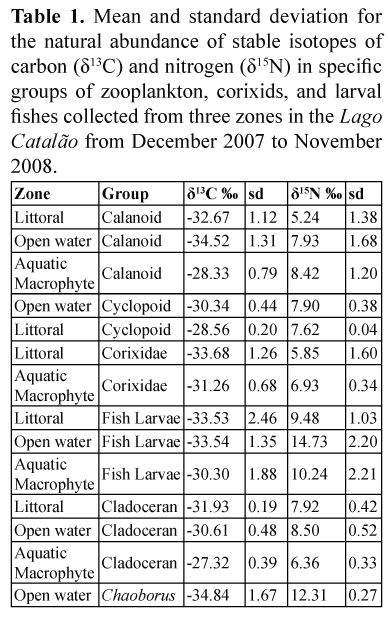
Samples of cladocerans, calanoid and cyclopoid copepods, insects of the family Corixidae, fish larvae and Chaoborus spp. (Table 1) were analysed separately for stable isotope ratios. Among them, the highest value of δ13C was registered for cladocerans from the aquatic macrophyte zone and the lowest value was registered for Chaoborus, sampled at night in the open-water zone. In terms of variation of δ13C within the same group, which reflects its trophic plasticity, cladocerans and calanoid copepods presented ranges of 4.61 and 4.34, respectively. Regarding δ15N, the highest values were observed for fish larvae from the open-water zone, and the lowest for calanoid copepods in the littoral zone. Fish larvae belonged to the Engraulididae and Characidae families, not including five specimens from the genus Pyrrulina (Lebiasinidae) sampled in August 2008 at the aquatic macrophytes zone, which presented δ13C values of -23.46 ± 0.04 and 4.82 ± 0.02.
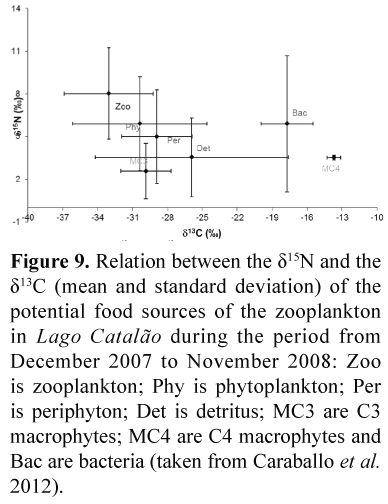
Values of δ13C and δ15N from different zooplankton feeding sources were taken from Caraballo et al. (2012) and are presented in Figure 9, showing that phytoplankton were the most 13C-depleted source, and the aquatic macrophytes the most enriched. Zooplankton tended to be more depleted in 13C than any of the sources, but not too far from phytoplankton. However, these are the mean values, which do not reflect the variation of such sources across the hydrological year.
DISCUSSION
The hydrological dynamics of Lago Catalão. In the complex hydrological cycle of a floodplain lake, gradients of temperature, light, conductivity, and nutrients interact affecting quality, quantity, and composition of zooplankton food sources. A rapid increment in the zooplankton richness is the regular result of the beginning of the process of flooding in tropical lakes (Brandorff & Andrade 1978, Twombly & Lewis 1989, Lindholm & Hessen 2007). The water input is associated with the increase of food resources (Torres-Bejarano et al. 2013), what is especially true in Lago Catalão, where the response of the macrophytes Echinochloa polystachya and Paspalum repens is immediate, covering broad areas that provide refuge for some organisms of the zooplankton (Hamilton 1990; Hamilton et al. 1992). Thus, the water input of the Rio Negro at the end of November begins the expansion of the trophic niches, which reaches its maximum in the flood, creating an inundated forest zone (locally called igapó) around the lake. In this process, the lake inundates the floodplain vegetation, including the wastes of the buffaloes that graze during the low waters, that has been associated with the increase of nutrient availability (Brito 2006).
Relative abundance of the Zooplankton. The exclusion of rotifers and copepod larval stages is undoubtedly one of the reasons why in our results there was a strong dominance of cladocerans at the three studied environments. It is possible to affirm, based on Trevisan & Forsberg (2007), that only 25% of the zooplankton community in terms of number of individuals was analysed, which corresponds to the larger sized organisms. In fact, the majority of the assessments of tropical zooplankton that include rotifers have shown them to be the dominant group in richness and abundance (Keppeler 2003). However, when counts of larval stages of the copepods were included, this group was found to have the highest abundance (Waichman et al. 2002).
Cladoceran dominance was more evident during the high water period in Lago Catalão. During the low water period, copepods showed an increase, and calanoids were the dominant group in the littoral zone during the isolation phase. Such changes may be associated with the presence of fish larvae at the littoral zone during low water resulting in a strong predation pressure upon cladocerans a situation observed by Leite et al. (2006) in the same lake. On the other hand, considering the abundance of calanoid copepods in this work.
In general, the distribution of zooplankton relative abundance in the Lago Catalãopresented an absolute dominance of two groups, mainly herbivorous, and a lower abundance of the cyclopoids, which are carnivorous zooplankters. Mesocyclops the genus of cyclopoid identified in Lago Catalão is known to feed on rotifers, cladocerans, nauplii of copepods, and protozoans (Williamson 1980). Carbon and nitrogen stable isotope ratios showed that the cladocerans Diaphanosoma spinolosum and Ceriodaphnia cornuta consumed predominantly phytoplankton (Caraballo et al. 2011), which had already been observed for the calanoid copepods by Yoshioka et al. (1994). The herbivorous efficiency of a zooplankton community is proportional to the mean size of the species presented (Matthews & Mazunder 2005b). For example, the variation in the feeding behavior of calanoid species may be higher than the difference in the same behavior between two species of Daphnia (Matthews 2005).
Based on analysis conducted in 2466 Norwegian lakes, Walseng et al. (2006) demonstrated that the microcrustaceans may be divided into three groups: strictly coastal-benthic species, species that are present at the coastal and pelagic regions, and a smaller group comprised of strictly pelagic species. This classification, although broad, does not encompass the characteristics of some large species, as those from the family Chydoridae, which normally are present in the samples collected within the aquatic macrophytes. Such large species are considered to inhabit littoral zones (Walseng et al. 2006), but are not present in our littoral zone samples, presumably because of high predation pressure by the fish larvae.
Seasonal variation in natural abundance of δ13C and δ15N. The analysis of seasonal variation of δ13C of the zooplankton from the different zones showed a more 13C-enriched mean value for samples collected from aquatic macrophytes (Figure 5), suggesting two sources of carbon, one originated from C3 plants, which generates more 13C-depleted values in the pelagic and coastal samples; and the other from C4 plants, which occur mainly in the aquatic macrophyte zone. Prior research carried out in the Amazon region considered the phytoplankton as C3 plants in addition to the C3 vascular plants, and the only significant C4 plants were considered to be the floating aquatic grasses, especially Paspalum repens and Echinochloa polystachya since they are the most abundant in biomass (Forsberg et al. 1993). However, an alternative explanation for the 13C-enriched zooplankton in the macrophyte zone would be a trophic linkage to attached algae (epiphyton), which are known to be variable in d13C but often relatively 13C-enriched (Hamilton et al. 1992).
The mesozooplankton consumes the phytoplankton, but also different groups of heterotrophic protists, which connects the microbial (detrital) trophic chain with the classical fish-zooplankton-algae trophic chain (Porter 1996). Generally, the copepods are considered efficient predators of ciliated microbes and the cladocerans efficient predators of bacteria (Perga et al. 2006), what could explain the patterns of δ13C of the zooplankton in the littoral and macrophyte zones obtained in this research. In the open-water zone, the values of δ13C of the zooplankton are closer to those of the phytoplankton, which agrees with the results of Oliveira et al. (2006). Green and chrysophyte algae of sizes smaller than 20 µm were the most abundant in the stomach content analysis of the fishes Argyrodiaptomus furcatus and Notodiaptomus iheringi, therefore algae were considered as an important source of food for these species in a reservoir at south-eastern Brazil (Rietzler et al. 2002). Therefore, it is possible to affirm that the mesozooplankton in Lago Catalãopresents high feeding plasticity, grazing over a δ13C range of 11.43, 8.01 and 9.95 in the littoral, open-water, and macrophyte zones, respectively. Of course, it may be the result of a punctual activity of a species (Carpenter et al. 2005, Matthews 2005), which would mask the inferences made about dietary sources. However, it is more likely that those species find the same resources in the three environments. Or it could be also the result of the intrinsic variability of the resource, as the result of the biogeochemical variation associated with the hydrological dynamics.
Except in May and July, when the lake experienced a cooling event, locally called "friagem" (Caraballo et al. 2014), the lowest values of δ13C were found during the months when copepods dominated (Fig. 4). Low values of δ13C may be associated with the consumption of methanogenic carbon (Bastviken et al. 2003) that has been widely demonstrated in the hypolimnion of floodplain lakes, as well as its input associated with the upper layers of the water column during the mixing events. The consumption of methanotrophic bacteria was linked to 13C depletion in consumers down to -50.3 by Kankaala et al. (2006). Thus, taking into account that the minimal values of δ13C for phytoplankton (-34 and -33 in the coastal and pelagic zones, respectively), the consumption of methanotrophic bacteria may be more important than has been considered, as suggested by Calheiros (2003) in her research at the Pantanal region. On the other hand, the degree of 13C depletion observed in zooplankton in this study could also be explained by consumption of 13C-depleted phytoplankton; such negative δ 13C values would be expected in waters high in dissolved carbon dioxide (Hamilton et al. 1992).
The values of zooplankton δ15N did not show high spatial variation, showing means of 6.88, 7.74 and 6.77 for the littoral, open-water, and macrophyte zones, respectively. The zooplankton δ15N did show variation associated with the flood pulse, with more 15N-depleted values during April and September, and more enriched values during December and July, corresponding with the hydrological phases. The variability of the values of δ15N suggested, assuming a trophic fractionation of 3.4 (Post 2002), that the zooplankton fed on just one trophic level. According to Matthews (2005), copepods are omnivorous, and therefore a higher value of δ15N is expected; however we did not observe 15N enrichment relative to the cladocerans.
Natural Abundance of δ13C and δ15N in relation to the hydrological phases. Zooplankton δ13C values from the aquatic macrophyte zone during the hydrological cycle of the lake were more 13C-enriched than values from the other two zones (Figure 5); however, within each zone they did not differ much across the three limnological phases (Figure 7). Besides particulate organic detritus from the C4 grasses, another source of C4 carbon may be heterotrophic bacteria that rely on C4 organic matter, the importance of which has been demonstrated by Waichman (1996) and Caraballo et al. (2012).
The mean values of zooplankton δ13C in the littoral and open-water zones showed similar degrees of 13C depletion during the filling and falling water phases, but became more 13C-enriched during the flood phase, which is considered the period of the year in which Lago Catalão (Almeida & Melo 2009) and generally the other floodplain lakes (Torres-Bejarano et al. 2013) become more homogeneous. During the high-water period, the lake is subject to a disturbance of variable magnitude and intensity caused by the "friagem", which mixes the water column, and can bring dissolved methane from the bottom waters to the surface waters (Caraballo et al. 2014). This mixing can in turn generating more 13C-depleted phytoplankton by the fixation of CO2 created from the oxidation of the isotopically lighter methane), as proposed by Kankaala et al. (2006). This phenomenon may have affected first the zooplankton of the open-water zone, and later, in the the coastal region during the falling water and isolation phases. The different dynamic of the macrophyte zone, could be explained if the mixing process by the cooling of the upper part of the column had been hindered by the plants.
In general, the mean values of δ15N were not significantly different (p > 0.05) for the three zones, but each showed similar seasonal variation (Figure 6). Is this seasonal variation a consequence of the seasonal change of the feeding habits of the zooplankton, or variation in the isotopic composition and structure of the resource? Or in other words, is it a trophic shift or just baseline isotopic variation? On the one hand, there is evidence that individual zooplankton species have the ability to exploit different resources, depending on the environmental conditions (Porter 1996). On the other hand, biogeochemical variability that could affect the isotopic composition of the food resources has been observed not only at seasonal time scale but also in diel cycles (Boëchat& Giani 2008). Seasonal variation in δ15N of Daphnia was defined by Lehmann et al. (2004) as dependent on the seasonal variation in δ15N of the dietary sources, which in turn varied as a consequence of the processes in the nitrogen cycle. The huge variability of the system and the trophic plasticity of mesozooplankton make it impossible to answer this question with the available information.
During the hydrological year studied, the phytoplankton had a mean δ13C value of -30.37 ± 5.79 including the variable contribution of the size fractions <60 µm, <30 µm and <10 µm (Caraballo et al. 2014). It is known that the δ13C of phytoplankton may vary with depth and with the size of cells. Even considering that cladocerans are efficient consumers of bacteria, Perga et al. (2006), based on the proportion of lipids of phytoplanktonic and bacterial origin, have not shown a significant contribution of bacterial C in the production of Daphnia. In this study, carbon and nitrogen stable isotope analysis showed that, independent of the pathways through which the carbon is circulating, the initial source of carbon and energy for the zooplankton community is mainly phytoplankton.
ACKOWLEDGEMENTS
Instituto Nacional de Pesquisas da Amazônia for logistical support and cooperation. The CTPETRO and PIATAM IV projects for financial support. The Universidad de Sucre, Colombia and Capes-Fapeamfor the scholarship and an anonymous referee, which comments strongly improve our work.
LITERATURE CITED
1. Almeida F.F. & S. Melo. 2009. Considerações limnológicas sobre um lago da planície de inundaçãoamazônica (Lago Catalão - Amazonas-Brasil). Revista Acta Scientiarum. Maringá 31(4): 387-395.
2. Araujo-Lima, C., B.R. Forsberg, R. Victoria & L.A. Martinelli. 1986. Energy sources for detritivorous fishes in the Amazon. Science 234: 1256-1258.
3. Bastviken, D., J. Ejlertsson, I. Sundh & L. Tranvik. 2003. Methane as a source of carbon and energy for lake pelagic food webs. Ecology 84: 969-981.
4. Boëchat, I. & A. Gianni. 2008. Seasonality affects diel cycles of seston biochemical composition in a tropical reservoir. Journal of Plankton Research 30(12): 1417-1430.
5. Brandorff, G.O. & E.R. Andrade. 1978. The relationship between the water level of the Amazon River and the fate of the zooplankton population in lago Jacaretinga, a várzea lake in the Central Amazon. Studies on neotropical Fauna and Environment 13: 63-70.
6. Brito, J.G. 2006. Influência do pulso de inundação sobre variáveislimnológicas de um lago de várzea da Amazônia Central, lago Catalão. Dissertação. Instituto Nacional de Pesquisas da Amazônia/FundaçãoUniversidade do Amazonas, Manaus. Brasil. 191pp.
7. Calheiros, D.F. 2003. Influência do pulso de inundaçãona composição isotópica (δ13C e δ15N) das fontes primárias de energia na planície de inundação do rio Paraguai (Pantanal MS). Tese de Doutorado. USP - Centro de Energia Nuclear na Agricultura, São Paulo. 164pp.
8. Caraballo, P. 1992. Historia de vida e dinâmicapopulacional de Daphnia gessneri e Ceriodaphnia cornuta (Crustacea-Cladocera) no Lago Calado, AM. Dissertação de Mestrado. Pós-graduação INPA/FUA, Manaus. 145pp.
9. Caraballo, P., A. Sanchez-Caraballo, B. Forsberg & R. Leite. 2011. Crescimento populacional e análise isotópica de Diaphanosoma spinolosum e Ceriodaphnia cornuta (Crustacea: Cladocera), alimentadas com diferentes frações de seston natural. Acta Scientiarum. Biological Sciences. Maringá. 33(1): 11-19.
10. Caraballo, P., B. Forsberg & R. Leite. 2012. Papel trófico del microbial loop en un lago de inundación en la Amazonia Central. Acta Biológica Colombiana 17(1): 103-116.
11. Caraballo, P., B.R. Forsberg, F.F.D. Almeida & R.G. Leite. 2014. Diel patterns of temperature, conductivity and dissolved oxygen in an Amazon floodplain lake: description of a friagem phenomenon. Acta Limnologica Brasiliensia, 26(3): 318-331.
12. Caraballo, P., B. Forsberg & R. Leite. 2014. Seasonal Variation in the Distribution and Isotopic Composition of Phytoplankton in an Amazon Floodplain Lake, Brazil. Acta BiológicaColombiana 19(2): 291-304.
13. Carpenter, S.R., M.L. Pace, J.J. Cole, M. Van de Bogert, D. Bade, D. Bastviken, R.M. Gille, J.R. Hodgson, J.F. Kitchell & E.S. Kritzberg. 2005. Ecosystem subsidies: terrestrial support of aquatic food webs from13C addition to contrasting lakes. Ecology 86: 2737-2750.
14. Coffin, R.B., J.P. Connolly & P.S. Harris. 1993. Availability of dissolved organic carbon to bacterioplankton examined by oxygen utilization. Marine Ecology Progress Series 101: 9-22.
15. Forsberg, B., C.A.R.M. Araujo-Lima, L.A. Martinellri, R. Victoria & J.A. Bonassi. 1993. Autotrophic carbon sources for fish of the central Amazon. Ecology 74(3): 643-652.
16. Hamilton, S.K. 1990. Zooplankton abundance and evidence for its reduction by macrophyte mats in two Orinoco floodplain lakes. J. Plankton Res. 12(2): 345-363.
17. Hamilton, S.K., W.M. Lewis Jr. & S.J. Sippel. 1992. Energy sources for aquatic animals in the Orinoco River floodplain: evidence from stable isotopes. Oecologia 89(3): 324-330.
18. Kankaala, P., S. Taipale, J. Grey, E. Sonninen, L. Arvola & R.I. Jones. 2006. Experimental 13C evidence for a contribution of methane to pelagic food webs in lakes. Limnology and Oceanography 51(6): 2281-2827.
19. Keppeler, E.C. 2003. Comparative study of the zooplankton composition lacustrine ecosystems in Southwestern Amazonia. Acta Scientiarum. Maringá 25(2): 467-477.
20. Lehmann, M.F.S.M., J.A. Bernasconi, A. Mckenzie, M. Barbieri & M. Veronesi. 2004. Seasonal variation of the δ13C and δ15N of particulate and dissolved carbon and nitrogen in Lake Lugano: Constraints on biogeochemical cycling in a eutrophic lake. Limnology and Oceanography 49: 415-429.
21. Leite, R., J.V. Silva & C.E. Freitas. 2006. Abundância e distribuição das larvas de peixes no Lago Catalão e no encontro dos rios Solimões e Negro, Amazonas, Brasil. Acta Amazonica 36(4): 557-562.
22. Lesack, L.F. 1988. Mass balance of nutrients, major solutes and water in an Amazon floodplain lake and biogeochemical implications for the Amazon Basin. PhD Thesis. University de California. Santa Barbara, USA. 484 p.
23. Lindholm, M. & D.O. Hessen. 2007. Zooplankton succession on seasonal floodplains: surfing on a wave of food. Hydrobiologia 592: 95-104.
24. Matthews, B. 2005. Food web ecology of zooplankton communities in lakes. PhD Thesis. University of Victoria, Victoria, Canada. 264p.
25. Matthews, B. & A. Mazumder. 2005b. Temporal variation in body composition (C:N) helps explain seasonal patterns of zooplankton δ13C. Freshwater Biology 50: 502-515.
26. Matthews, B. & A. Mazumder. 2005c. Consequences of large temporal variability of zooplankton δ15N for estimates of fish trophic variation. Limnology and Oceanography 50: 1404-1414.
27. Matthews, B. & A. Mazumder. 2006. Habitat specialization and the exploitation of allochthonous carbon by zooplankton. Ecology 87: 2800-2812.
28. Mazumder, A. 1994. Patterns of algal biomass in dominant odd- vs. even-link lake ecosystems. Ecology 75:1141-1149.
29. Oliveira, A.C., M.G. Soares, L. Martinelli & M.Z. Moreira. 2006. Carbon sources of fish in an Amazonian floodplain lake. Aquat. Sci. 68: 229-238.
30. Perga, M.E., M. Kainz, B. Matthews & A. Mazumder. 2006. Carbon pathways to zooplankton: insights from the combined use of stable isotope and fatty acid biomarker. Freshwater Biology 51: 2041-2051.
31. Pomeroy, L.R., P.J. Williams, F. Azam & J.E. Hobbie. 2007. The microbial loop. Oceanography 20(2): 28-33.
32. Porter, K. 1996. Integrating the microbial loop and the classic grazer food chain into a realistic planktonic food web. En: G. Polis & K.O. Winemiller (eds). Food webs: Integration of patterns and dynamics: 51-59. Chapman and Hall. New York.
33. Post, D.M. 2002. Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology 83: 703-718.
34. Rejas, D., S. Declerkck, J. Auwerkerken, P. Tak & L. Meester. 2005. Plankton dynamics in a tropical floodplain lake: fish, nutrients, and the relative importance of bottom-up and top-down control. Freshwater Biology 50: 52-69.
35. Rietzler, A.C., J.G. Tundisi & T. Matsumura-Tundisi. 2002. Life cycle, feeding and adaptive strategy implication of the co-occurrence of Argyrodiaptomus furcatus and Notodiaptomus iheringi in Lobo-Broa reservoir (SP, Brazil). Braziliam Journal of Biology 62(1): 93-105.
36. Santos, N.R., E.J.G. Ferreira & S. Amadio. 2007. Effect of seasonality and trophic group on energy acquisition in Amazonian fish specie. Ecology of Freshwater Fish 17: 340-348.
37. Sieburth, J.M.C.N., V. Smetacek & J. Lenz. 1978. Pelagic ecosystem structure: heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnology and Oceanography 23: 1256-1263.
38. Smyntek, P.M., M.A. Teece, K.L. Schulz & S.J. Thackeray. 2007. A standard protocol for stable isotope analysis of zooplankton in aquatic food web research using mass balance correction models. Limnology and Oceanography 52(5): 2135-2146.
39. Torres-Bejarano, A.M., Duque, S.R. & P.R. Caraballo-Gracia. 2013. Heterogeneidad espacial y temporal de las condiciones físicas y químicas de dos lagos de inundación en la amazonia colombiana. Actualidades Biológicas 35(98): 63-76.
40. Trevisan, G. & B.R. Forsberg. 2007. Relationships among nitrogen and total phosphorus, algal biomass and zooplankton density in the central Amazonia lakes. Hydrobiologia 586: 357-365.
41. Twombly, S. & W.M. Jr Lewis. 1987. Zooplankton abundance and species composition in Laguna la Orsinera, a Venezuelan floodplain lake. Arch. Hydrobiol. Suppl. 79 (1): 87-107.
42. Twombly, S. & W.M. Jr Lewis. 1989. Factors regulating cladoceran dynamics in a Venezuelan floodplain lake. Journal of Plankton Research 11: 317-333.
43. Van der Zanden, M.J. & J.B. Rasmussen. 1996. A trophic position model of pelagic food webs: impact on contaminant bioaccumulation in lake trout. Ecological Monographs 66: 451-477.
44. Waichman, A.V. 1996. Autotrophic carbon sources for heterotrophic bacterioplankton in a floodplain lake. Hydrobiologia 341: 27-36.
45. Waichman, A., C. Garcia-Davila, E. Hardy & B. Robertson. 2002. Composição do Zooplânctonem diferentes ambientes do Lago Camaleão, na Ilha da Marchantaria, Amazonas, Brasil. Acta Amazonica 32(2): 339-347.
46. Walseng, B., D.O. Hessen, G. Halvorsen & A.K. Schartau. 2006. Major contribution from littoral crustaceans to zooplankton species richness in lakes. Limnology and Oceanography 51(6): 2600-2606.
47. Williamson, C.E. 1980. The predatory behavior of Mesocyclops edax: Predator preferences, prey defenses, and starvation-induced changes. Limnology and Oceanography 25: 903-909.
48. Yoshioka, T., E. Wada & H. Hayashi. 1994. A stable isotope study on seasonal food web dynamic in an eutrophic lake. Ecology 75(3): 835-846.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Camilo Andrade-Sossa, Juan Pablo Alvarez-Silva, Nelson Aranguren-Riaño, Osvar Sterling Cupitra-Gómez, Silvia Lucía Villabona-González, Angélica M. Torres-Bejarano, Carlos López. (2023). Zooplankton studies in Colombian fresh and brackish water ecosystems: A review and future perspectives. Limnologica, 100, p.126081. https://doi.org/10.1016/j.limno.2023.126081.
2. Ryota Nakajima, Elvis V. Rimachi, Edinaldo N. Santos-Silva, Laura S.F. Calixto, Rosseval G. Leite, Adi Khen, Tetsuo Yamane, Anthony I. Mazeroll, Jomber C. Inuma, Erika Y.K. Utumi, Akira Tanaka. (2017). The density and biomass of mesozooplankton and ichthyoplankton in the Negro and the Amazon Rivers during the rainy season: the ecological importance of the confluence boundary. PeerJ, 5, p.e3308. https://doi.org/10.7717/peerj.3308.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 Caldasia

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).






















