Basic growth analysis in strawberry plants (Fragaria sp.) exposed to different radiation environments
Keywords:
sombra, espectro lumínico, cantidad de luz, fotomorfogénesis, coberturas de color (es)Downloads
Este estudio se orientó a establecer si la cantidad y la calidad de la luz afectan el crecimiento y desarrollo de las plantas de fresa. Las plantas se desarrollaron en un invernadero en Tunja / Colombia, bajo diferente calidad de luz (amarilla, verde, azul, transparente, roja y control sin cobertura de color), proporcionada por películas de polipropileno. Los filtros coloreados aportaron también diferentes niveles de sombreado a las plantas. Se calcularon los índices comúnmente utilizados para el análisis básico del crecimiento en vegetales. Los tratamientos se colocaron en un diseño en bloques al azar con diez replicaciones. Los filtros se ubicaron 1m sobre el follaje del cultivo, desde el trasplante hasta la cosecha de las plantas. La tasa de asimilación neta se redujo con las coberturas de color con excepción de la cobertura transparente y el control. La cobertura verde indujo un incremento en la relación de área foliar, la relación raíz : vástago, la relación de peso foliar y en el área foliar específica; sin embargo, el índice de cosecha, las tasa absoluta y relativa de crecimiento se redujeron en plantas que crecieron bajo esta cobertura. Las plantas expuestas a la película amarilla mostraron el mayor índice de cosecha. La respuesta de las plantas de fresa en relación con las tasas de crecimiento fueron la consecuencia del efecto conjunto de la cantidad y la calidad de la luz incidente.The present study sought to understand how quantity and quality of light affect growth and development in strawberry plants. Plants were grown in a greenhouse in Tunja, Colombia, under different light quality regimes provided by polypropylene films (yellow, green, blue, transparent, red, and a control without plastic film cover). These colored filters also provided different shading levels to plants. The authors measured growth parameters and calculated various indices commonly used in basic plant growth analysis. Plastic light filters were placed 1 m above crop foliage and were kept in place from initial transplanting until final harvest. Net assimilation rate was reduced under colored filters, but not under the transparent film or the film-free control. Green cover induced an increase in leaf area ratio, root to shoot ratio, leaf weight ratio, and specific leaf area. Harvest index and absolute and relative growth rate were reduced in plants grown under green film. The growth response of strawberry plants was the consequence of the combined effect of light quantity and quality. Results also showed the striking influence of green light on strawberry growth.
CROP PHISIOLOGY
1 Plant Ecophysiology Research Group, Faculty of Agricultural Sciences, Universidad Pedagógica y Tecnológica de Colombia (UPTC). Tunja (Colombia).
2 Division Urban Plant Ecophysiology, Faculty for Agriculture and Horticulture, Humboldt-Universität zu Berlin. Berlin (Germany).
3 Corresponding author. fanor.casierra@uptc.edu.co
Received for publication: 20 July, 2011. Accepted for publication: 1 March, 2012.
ABSTRACT
The present study sought to understand how quantity and quality of light affect growth and development in strawberry plants. Plants were grown in a greenhouse in Tunja, Colombia, under different light quality regimes provided by polypropylene films (yellow, green, blue, transparent, red, and a control without plastic film cover). These colored filters also provided different shading levels to plants. The authors measured growth parameters and calculated various indices commonly used in basic plant growth analysis. Plastic light filters were placed 1 m above crop foliage and were kept in place from initial transplanting until final harvest. Net assimilation rate was reduced under colored filters, but not under the transparent film or the film-free control. Green cover induced an increase in leaf area ratio, root to shoot ratio, leaf weight ratio, and specific leaf area. Harvest index and absolute and relative growth rate were reduced in plants grown under green film. The growth response of strawberry plants was the consequence of the combined effect of light quantity and quality. Results also showed the striking influence of green light on strawberry growth.
Key words: shading, light spectrum, light quantity, photomorphogenesis, colored films.
RESUMEN
Este estudio se orientó a establecer cómo la cantidad y la calidad de la luz afectan el crecimiento y desarrollo de las plantas de fresa. Las plantas se desarrollaron en un invernadero en Tunja (Colombia), bajo diferentes calidades de luz (amarilla, verde, azul, transparente, roja y control sin cobertura de color), proporcionadas por películas de polipropileno. Los filtros coloreados aportaron también diferentes niveles de sombreado a las plantas. Se calcularon los índices comúnmente utilizados para el análisis básico del crecimiento en vegetales. Los filtros se ubicaron 1 m sobre el follaje del cultivo, desde el trasplante hasta la cosecha de las plantas. La tasa de asimilación neta se redujo con las coberturas de color con excepción de la cobertura transparente y el control. La cobertura verde indujo un incremento en la relación de área foliar, la relación raíz:vástago, la relación de peso foliar y en el área foliar específica; sin embargo, el índice de cosecha, las tasas absoluta y relativa de crecimiento se redujeron en plantas que crecieron bajo esta cobertura. La respuesta de las plantas de fresa en relación con las tasas de crecimiento fue la consecuencia del efecto conjunto de la cantidad y la calidad de la luz incidente. Los resultados mostraron un fuerte efecto de la luz verde sobre el crecimiento de plantas de fresa.
Palabras clave: sombra, espectro lumínico, cantidad de luz, fotomorfogénesis, coberturas de color.
Introduction
Growth analysis is a widely-used tool in research areas ranging from plant breeding to crop physiology to plant ecology (Poorter and Garnier, 1996). Growth analysis represents the first step in analysis of primary productivity, which makes it an important link between the measurement of crop yield and the understanding of physiological phenomena that determine yield. A major advantage of growth analysis lies in the ease of measuring the raw data on which it is based, such as dry plant weight, leaf area, and time (Santos-Castellanos et al., 2010). Detailed plant growth analysis permits researchers to quantify aspects such as the duration of the plant life cycle, the definition of phenological and developmental stages, and the distribution of assimilates in different organs (Azofeifa and Moreira, 2004). Furthermore, growth analysis is essential to achieving a better understanding of the physiological processes that define plant production. In this respect it serves to define the best crop management alternatives in terms of fertilization, irrigation, phytosanitary practices, pruning, and planting arrangement and density, among other things (Lambers and Poorter, 1992).
Solar radiation is the energy source used by plants in the process of photosynthesis, which is the means by which plant matter is produced. Part of this plant matter is the harvested crop (Hernández et al., 2001). Li et al. (2010) affirm that strawberry yield has a negative correlation with solar radiation, which suggests that high solar radiation and high temperature (associated with water loss) can induce a negative response in strawberry plants, with a consequent decrease in fruit formation. The optimization of plant use of resources such as fertilizer depends in large part on the quality of solar radiation received by plants, which exhibit higher or lower production as a function of this radiation. On the high plains of central Colombia, 6.3% of total production costs for small farmers producing strawberry on a 20-month cycle consist in fertilizers and soil conditioners, while in Antioquia these inputs represent 7.7% of total production costs (Agronet, 2009). If photosynthetic efficiency could be improved by changing the light wavelengths to which plants are exposed so as to promote fruit production, it would be possible to increase yield using the same level of inputs (Hernández et al., 2001; Patil et al., 2001; Casierra-Posada and Rojas, 2009). Plant response to different colors of light has been documented by various authors (Casierra-Posada and Rojas, 2009). Inoue et al. (2008) and Hoang et al. (2008) reported changes in morphological and physiological behavior in Arabidopsis sp. plants under the influence of blue and red light. These plant responses are mediated by cryptochromes and phytochromes, and are related to the regulation of leaf position and other processes related to light capture. In this same line, farmers have used colored covers to in- crease yield and growth in different plant species. The use of red and blue polyshade mesh have an effect on growth and flowering on different cultivars of Phalaenopsis sp.; red mesh induced precocity in the majority of accessions evaluated, while plants growing under blue mesh developed higher leaf area (Leite et al., 2008). In the spirit of offering farmers new cropping alternatives that increase total yield, the objective of the present study was to evaluate the effect of different colored coverings on basic growth parameters in greenhouse-grown strawberry plants.
Materials and methods
The experiment was carried out in Tunja, Colombia, under glass greenhouse conditions. Ten plants per treatment were subjected to solar radiation filtered through 15 µm-thick polypropylene films of different colors: red, yellow, blue, green, and transparent. Control plants grew in the greenhouse without any polypropylene covering. Photosynthetically active radiation (PAR) and light reduction (opacity) registered beneath the different covers are shown in Tab. 1. Average temperature inside the greenhouse was 15.8°C, with 72% relative humidity.

Planting material consisted of strawberry plantlets (Fragaria sp. var. Chandler) previously exposed to stratifying temperatures of 4±1°C during three weeks. After this period they were placed in glass jars containing a nutrient solution with the following composition in mg L-1: nitric nitrogen 40.3; ammonium nitrogen 4.0; phosphorus 20.4; potassium 50.6; calcium 28.8; magnesium 11.4; sulfur 1.0; iron 1.12; manganese 0.112; copper 0.012; zinc 0.0264; boron 0.106; molybdenum 0.0012; cobalt 0.00036.
Frames were covered with polyethylene film of the different experimental colors and fitted over the glass jars on tables in the greenhouse, each frame covering 10 plants at a height of 1 m above the plants. The greenhouse was equipped with piping and hoses connected to an aeration system to oxygenate the plants growing in glass containers. At the beginning of the experiment dry weight was measured in 10 plantlets to give an initial value for the calculation of growth indices, according to the methodology reported by Hunt (1990).
Treatments were arranged in a completely randomized design with 10 replications. Results were subjected to analysis of variance (ANOVA) and treatments were compared using Tukey's range test with a significance level of 5%. Statistical analyses were performed with version 19.0.0 of the IBM®-SPSS statistics program (Statistical Product and Service Solutions, IBM Corporation, New York, NY).
Results and discussion
Net assimilation rate (NAr)
Significant differences were found (P≤0.05) in the values of NAR. Plants grown under blue, red, green, and yellow films had NAR 64.52, 49.32, 78.66, and 43,76% lower than control plants grown with no colored cover (Fig. 1).

It should be pointed out that maximum absorption ranges for chlorophyll occur in the blue-violet range (400-500 nm) and the orange-red range (600-700 nm) of the visible spectrum (Mc Donald, 2003), which would explain the behavior of NAR in plants grown under yellow, blue, and red films as compared to green films. Plants exposed to these colors would be favored by a higher absorption of quanta by chlorophyll.
When radiation intensity is low, plants invest little in production of photosynthesis-related enzymes (Lambers et al., 1998). Hence in low light conditions, as in the case of plants developing under colored films, morphology and architecture of aerial plant parts become relatively more important; according to Evans et al. (1988) leaf area ratio is maximized in such conditions, but there are limitations to maximizing net assimilation rate. However, this general tendency does not explain the difference in NAR between green and red films, which were almost equally opaque.
Svenson (1993) placed strawberry plants in either green or white pots and exposed them to differing degrees of shade. He found that the reflection of light from different pot colors alone did not influence dry weight of crowns and leaves, but that a 60% shading combined with white pot color notably reduced dry weight of aerial plant parts and fruits, as compared to the same shading level in green pots. It can be inferred then that the extremely low NAR value in the present study for plants grown under green film was due to the combined action of the shading caused by the film (73.70%) and low absorption of green light by plants, given that the majority of photons in this wavelength range are reflected as diffuse radiation (Lazo and Ascencio, 2010). Furthermore, if we consider that net assimilation rate is a measure of average efficiency of plant leaves, or an indirect measurement of the net gain of assimilates per unit of leaf area over time (Brown, 1984), then treatments with higher light levels such as the control and the transparent film should trigger higher assimilate production than in plants grown under the colored covers. In the present study control plants without plastic film or those grown under transparent film presented higher NAR values, which agrees with this affirmation. However, the results of an experiment carried out by Casierra-Posada and Rojas (2009) showed that broccoli plants exposed to red covers showed better results in terms of total dry matter production. This implies that photomorphogenic responses differ according to plant species.
Root to shoot ratio
The root to shoot ratio of plants under green cover presented significantly higher values (86.77% higher) as compared to control plants with no cover. All other cover colors showed no significant difference with the control treatment (Fig. 2).

Increased assimilate allocation towards leaves as a response to shading normally coincides with a reduction in root dry matter (Björkman, 1981). This was not the case in the present study's green cover treatment, given that root to shoot ratio rose as compared to the other treatments, despite the green polyethylene film's shading 73.70% of incident light. The red film, which had an opacity of 71.01%, similar to the green film, did not however demonstrate the same increase in root to shoot ratio. This suggests that this parameter was more influenced by light than by shading.
As a point of comparison, Antonious and Kasperbauer (2002) placed colored panels on the soil surface in a carrot crop and measured changes in root to shoot ratio as a result of light reflected from the panels onto leaves. They found differences only in plants exposed to white panels as compared to the other panel colors (blue, green, and red), which contrasts with the present experiment, in which the green treatment was that which showed differences as compared to all others. The use of mulches on the soil surface obviously modifies the physico-chemical conditions in the soil by raising temperature, but mulches also trigger morpho-physiological changes in plants, due primarily to the reflection of certain light wavelengths from the mulch onto leaves, which can in turn increase root proliferation and thus improve growth and development (Solaiman et al., 2008).
Plants must balance biomass assignation to leaves, which increases the capacity to capture light and carbon dioxide and hence improves growth rate, and assignation to roots, which allows increased water and nutrient capture from the soil at the expense of aboveground growth. From an ecological viewpoint, a plant with more biomass allocated to its roots, as was the case in the strawberry plants growing under green cover in the present experiment, would exhibit slower growth but would possess certain survival advantages in a resource-limited environment (Castro- Diez, 2002).
Leaf area ratio (LAR)
Compared to control plants, plants exposed to yellow, blue, red, and green covers showed significantly higher LAR values 73.12, 169.89, 96.05, and 320.60%, respectively (Fig. 3).

The productive capacity of a plant depends among other things on leaf expansion and the distribution of photosynthetically active versus inactive tissues (Puntieri and Gómez, 1988). The maximization of leaf area relative to biomass (LAR) can be achieved by increased leaf expansion in space, an almost universal mechanism (Björkman, 1981). Different results in different environments, as seen in the present study, could be due to different shading levels among treatments (Tab. 1), but nevertheless the differences in the present study are striking between strawberry plants grown under red and green films. These films exhibited 71.01 and 73.70% shading, respectively, which is surely too minor a difference to explain the large contrast in LAR values under these two treatments, especially considering that the much less opaque yellow film gave similar results to the red film in this parameter. Without totally discounting the possibility of different shading levels influencing results, film color must be considered as the primary factor explaining the differences in LAR.
As mentioned before, under low-light conditions plants do not maximize production of photosynthetic enzymes, so to increase photosynthesis they must rely on changes in aboveground morphology and architecture (Lambers et al. 1998). In other words, LAR is maximized under low light, but NAR cannot be. This would explain why plants show high LAR and low NAR grown under very opaque green cover, though it fails to account for the different results under almost equally opaque red film.
Leaf weight ratio
As occurred with LAR, LWR showed highly significant differences (P≤0.05) between the control treatment and all other treatments save the clear plastic. Compared to control plants, those exposed to yellow, blue, red, and green covers showed values 23.29, 59.43, 33.89, and 113.32% higher, respectively, for this parameter (Fig. 4).

Many plant species exhibit an increase in leaf weight ratio as one type of adaptation response to shaded conditions (Björkman, 1981). In the present study, LWR increased under light-excluding plastic films as compared to control plants grown without film. In other words, biomass was increasingly allocated to the formation of assimilatory surface. This was especially true in the case of the green cover, which again indicates the importance of light quality in addition to light quantity in different growth parameters.
Harvest index (HI)
The green cover induced a 91.34% reduction in HI (the value of harvestable dry matter) as compared to control plants grown without colored filters (Fig. 5). Regarding this parameter, Waterer et al. (2001) evaluated the response of different horticultural crops such as pepper, tomato, squash, and melon exposed to transparent, red, blue, yellow, silver, white, and black mulches and found that plant response to the light reflected from mulches varied widely with the species and the moment of measurement. In 2000, red and blue mulches promoted growth and yield as compared to other mulch colors. In 2001, on the other hand, the more reflective mulches (blue and silver) induced higher yields. Franquera (2011) affirms that colored plastic mulches affect soil temperature, but that they also serve to reflect red and far-red light that influence phytochromes to increase plant growth and yield. With respect to the results of the present study, the photomorphogenic response of plants in terms of harvest index depends on the quality of incident light. However, the effect of light quantity should not be ignored. Together these factors would produce modifications in crop yield, given that plant morphogenesis (the form in which plants grow and develop) varies according to the spectrum and total quantity of light, which change due to environmental factors (Bonser and Aarssen, 2003).
Absolute growth rate (AGR) Absolute growth rate showed significant differences between control plants and those grown under yellow, blue, and green filters, which respectively presented values 15.11, 47.06, and 85.63% lower than control plants grown without plastic covers (Fig. 6).

The behavior of AGR can be explained by the fact that plants absorb photons in the red and blue range of the spectrum, while absorption of light in the green and far-red ranges is weaker, and much of these photons are reflected by plants as diffuse radiation (Lazo and Ascencio, 2010). Furthermore, a low level of photosynthetically active radiation and a low proportion of red to far-red light promote apical dominance and in some species promote internode lengthening (Casierra-Posada et al., 2012).
Relative growth rate (RGR)
Plants grown under blue and green films showed RGR values 18.03 and 47.37% lower, respectively, than control plants without cover (Fig. 7).

Regarding the AGR and RGR values found in the present study, Rikvin (1989) and Senger (1987) described faster growth and higher protein and chlorophyll content in plants exposed to blue light as compared to those grown under white light. Nevertheless, other authors suggest that light level is more important than spectral composition, and that after acclimatization plant responses depend on available light quantity and not quality (Gostan et al., 1986; Morel et al., 1987; Humbeck et al., 1988). The present study would support the first affirmation as opposed to the second, since red and green films allowed through roughly equal amounts of light, but growth rates were very different between these two treatments.
Specific leaf area (SLA) Plants exposed to yellow, blue, red, and green filters showed SLA values 40.29, 70.04, 46.24, and 97.57% higher than control plants (Fig. 8).
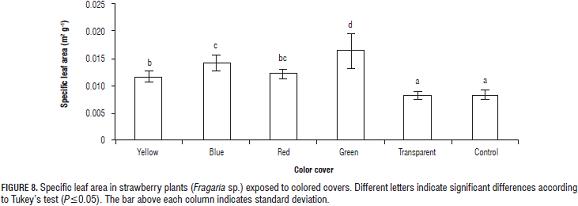
Research reported by Björkman (1981) shows that plants can adjust to low-light environments by increasing SLA, which is to say that they increase leaf area per unit of leaf weight, making for thinner, larger leaves. Despite this, in some species the increase in dry matter allocated to leaves (leaf weight ratio) is less pronounced than the effect on specific leaf area (Páez et al., 2000). This was not the case in the present study, since both SLA and LWR increased in a proportionally similar manner.
Conclusions
Most plant species respond to differences in light quality (color or wavelength) and light quantity (density of photon flux or irradiance), as well as to combinations of these two factors (Lazo and Ascencio, 2010).
Miranda and Williams (2007) evaluated photochemical efficiency of photosystem II (PSII) in vitro in strawberry leaves, and found an increase in this parameter from 0.64 under white light to 0.80 under yellow light. Furthermore, leaves developed under blue light were similar to those grown under white light in many parameters related to chlorophyll fluorescence, except for the initial fluorescence level. Casierra-Posada and Peña-Olmos (2012) exposed strawberry plants to different colored covers and found that different light quality influenced chlorophyll content. They also found that chlorophyll a concentration was higher in leaves growing under green and red light, followed by leaves in the blue, transparent, and yellow treatments. Wang et al. (2009) exposed Cucumis sativus to different colors of light, and found that all plants grown under monochromatic light showed reduced growth, CO2 assimilation rate, and PSII quantum yield (FPS II) as compared to plants grown under white light. The reduction in these parameters was most pronounced in plants grown under yellow, red, and green light. Furthermore, the reduction in ΦFPS II was due primarily to a reduction in photochemical quenching.
The present study confirmed the importance of both light quantity and light quality in plant growth. The blue and far-red ranges of the light spectrum are known for their important roles in genetic expression and in morphogenesis (Reymond et al., 1992; Kaufman, 1993; Short et al., 1994; Gupta and Tripathy 2010), but the present study clearly showed the important, often negative effective of green light on growth in strawberry.
Acknowledgements
This study was undertaken with support from the Directorate of Research (DIN) of the Universidad Pedagógica y Tecnológica de Colombia (Tunja) and from Colciencias through their young researcher program, as well as with support from the Plant Ecophysiology research group within the Agronomic Engineering program of the Faculty of Agricultural Sciences.
Literature cited
Agronet. 2009. Costos de producción por hectárea de fresa en la región Cundiboyacense y Antioquia. In: SIPSA, http://www.agronet.gov.co/www/htm3b/public/boletines/Costos2009trim1/Agricolas/Cundiboyacense/EC%20Fresa-mediano.pdf; http://www.agronet.gov.co/www/htm3b/public/boletines/Costos2009trim1/Agricolas/Antioquia/EC%20Fresapeque%C3%B1o.pdf; consulted: March, 2012.
Antonious, G.F. and M.J. Kasperbauer. 2002. Color of light reflected to leaves modifies nutrient content of carrot roots. Crop Sci. 42, 1211-1216.
Azofeifa, A. and M. Moreira. 2004. Análisis de crecimiento del chile jalapeño (Capsicum annuum L. cv. hot), en Alajuela, Costa Rica. Agron. Costarr. 28(1), 57-67.
Björkman, O. 1981. Responses to different quantum flux densities. pp. 57-107. In: Lange, O.L., P.S. Nobel, C.B. Osmond, and H. Ziegler (eds.). Physiological plant ecology. I. Responses to the physical environment. Springer-Verlag. Encycl. Plant Physiol. New Ser. Vol. 12A. New York, NY.
Bonser, S.P. and L.W. Aarssen. 2003. Allometry and development in herbaceous plants: functional responses of meristem allocation to light and nutrient availability. Amer. J. Bot. 90(3), 404-412.
Brown, R.H. 1984. Growth of the green plant. pp. 153-174. In: Tesar, M.B. (ed.). Physiological basis of crop growth and development. American Society of Agronomy, Madison, WI.
Casierra-Posada, F. and J.F. Rojas. 2009. Efecto de la exposición del semillero a coberturas de colores sobre el desarrollo y productividad del brócoli (Brassica oleracea var. italica). Agron. Colomb. 27(1), 49-55.
Casierra-Posada, F. and J.E. Peña-Olmos. 2012. Contenido de pigmentos en hojas de fresa (Fragaria sp.) expuestas a diferente calidad espectral. In press.
Casierra-Posada, F., P. Nieto, and C. Ulrichs. 2012. Crecimiento, producción y calidad de flores en calas (Zantedeschia aethiopica (L.) K. Spreng) expuestas a diferente calidad de luz. Rev. UDCA Actual. Divulg. Cient. 15 (1), 97-105.
Castro-Diez, P. 2002. Factores que limitan el crecimiento de la vegetación leñosa mediterránea. Respuestas de las plantas: de órgano a comunidad. pp. 47-85. In: Charco, J. (ed.). La regeneración natural del bosque mediterráneo en la península ibérica. ARBA; Ministerio de Medio Ambiente, Madrid.
Evans, J.R., S. Von Caemmerer, and W.W. Adams III. 1988. Ecology of photosynthesis in sun and shade. CSIRO, Melbourne, Australia.
Franquera, E.N. 2011. Influence of different colored plastic mulch on the growth of lettuce (Lactuca sativa). J. Ornam. Hort. Plants 1(2), 97-104.
Gostan, J., C. Lechuga-Deveze, and L. Lazzarra. 1986. Does blue light affect the growth of Chaetoceros protuberans (Bacillariophyceae). J. Phycol. 22, 63-71.
Gupta, V. and B.C. Tripathy. 2010. Effect of light quality on chlorophyll accumulation and protein expression in wheat (Triti- cum aestivum L.) seedlings. Int. J. Biotechnol. Biochem. 6(4), 521-536.
Hoang, N., J.P. Bouly, and M. Ahmad. 2008. Evidence of a lightsensing role for folate in Arabidopsis cryptochrome blue-light receptors. Mol. Plant 1(1), 68-74.
Hernández, J., I. Escobar, and N. Castilla. 2001. La radiación solar en invernaderos mediterráneos. Horticultura 157, 18-27.
Humbeck, K., B. Hoffman, and H. Senger. 1988. Influence of energy flux and quality of light on the molecular organization of the phytoplankton apparatus in Scenedesmus. Planta 173, 205-212.
Hunt, R. 1990. Basic growth analysis. Plant growth analysis for beginners. Unwin Hyman, Boston, MA.
Inoue, S., T. Kinoshita, A. Takemiya, M. Doi, and K. Shimazaki. 2008. Leaf positioning of Arabidopsis in response to blue light. Mol. Plant 1(1), 15-26.
Kaufman, L.S. 1993. Transduction of blue-light signals. Plant Physiol. 102, 333-337.
Lambers, H., F.S. Chapin III, and T.L. Pons. 1998. Plant physiological ecology. Springer, New York, NY.
Lambers, H. and H. Poorter. 1992. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 23, 187-261.
Lazo, J.V. and J. Ascencio. 2010. Efecto de diferentes calidades de luz sobre el crecimiento de Cyperus rotundus. Bioagro 22(2), 153-158.
Leite, C.A., R.M. Ito, G.T. Lee, R. Ganelevin, and M.Â. Fagnani. 2008. Light spectrum management using colored nets aiming to controlling the growth and the blooming of Phalaenopsis sp. Acta Hort. 770, 177-184.
Li, H., T. Li, R.J. Gordon, S.K. Asiedu, and K. Hu. 2010. Strawberry plant fruiting efficiency and its correlation with solar irradiance, temperature and reflectance water index variation. Environ. Exp. Bot. 68, 165-174.
Mc Donald, M.S. 2003. Photobiology of higher plants. John Wiley & Sons, West Sussex, UK.
Miranda, J. and R. Williams. 2007. Developmental influence of in vitro light quality and carbon dioxide on photochemical efficiency of PS II of strawberry leaves (Fragaria x ananassa). J. Appl. Hort. 9(1), 13-16.
Morel, A., L. Lazzarra, and G. Gostan. 1987. Growth rate and quantum yield time response for a diatom to changing irradiances (energy and color). Limno Oceanogr. 32, 1066-1084.
Páez, A., V. Paz, and J.C. López. 2000. Crecimiento y respuestas fisiológicas de plantas de tomate cv. Río Grande en la época mayo-julio. Efecto del sombreado. Rev. Fac. Agron. (LUZ) 17, 173-184.
Patil, G.G., R. Oi, A. Gissinger, and R. Moe. 2001. Plant morphology is affected by light quality selective plastic films and alternating day and night temperature. Gartenbauwiss. 66(2), 53-60.
Poorter, H. and E. Garnier. 1996. Plant growth analysis: an evaluation of experimental design and computational methods. J. Exp. Bot. 47(302), 1343-1351.
Puntieri, J.G. and I.A. Gómez. 1988. Análisis del crecimiento del almancay (Alstroemeria aurantiaca D. Don) en dos poblaciones naturales. Rev. Chil. Hist. Nat. 61, 177-185.
Reymond, P., T.W. Short, W.R. Briggs, and K.L. Poff. 1992. Lightinduced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA 89(10), 4718-4721.
Rikvin, R.B. 1989. Influence of irradiance and spectral quality on the carbon metabolism of phytoplankton. I. Photosynthesis, chemical composition and growth. Mar. Ecol. Proc. Ser. 55, 291-294.
Santos-Castellanos, M., M. Segura-Abril, and C.E. Ñústez-López. 2010. Análisis de crecimiento y relación fuente-demanda de cuatro variedades de papa (Solanum tuberosum L.) en el municipio de Zipaquirá (Cundinamarca, Colombia). Rev. Fac. Nal. Agr. Medellín 63(1), 5253-5266.
Senger, H. 1987. Blue light responses: phenomena and occurrence in plants and microorganisms.Vol. I. and Vol II. CRC Press, Boca Raton, FL.
Short, T.W., M. Porst, J. Palmer, E. Fernbach, and W.R. Briggs. 1994. Blue light induces phosphorylation at seryl residues on a pea (Pisum sativum L.) plasma membrane protein. Plant Physiol. 104, 1317-1324.
Solaiman, A.H.M., M.H. Kabir, A.M.F. Jamal-Uddin, and M. Hasanuzzaman. 2008. Black plastic mulch on flower production and petal coloration of aster (Callistephus chinensis). Amer. Euras. J. Bot. 1, 5-8.
Svenson, S.E. 1993. Shading and pot color influence growth and flowering of strawberry firetails. Proc. Fla. State Hort. Soc. 106, 286-288.
Wang, H., M. Gu, J. Cui, K. Shi, Y. Zhou, and J. Yu. 2009. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B. 96, 30-37.
Waterer, D., J. Bantle, and K. Leray. 2001. Vegetable cultivar and cultural trials 2001. In: University of Saskatchewan, Agriculture Development Fund & Agri-Food Innovation Fund, http://www.usask.ca/agriculture/plantsci/vegetable/resources/publication/2010resources/Book%20Color.pdf; consulted: March, 2012.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2012 Agronomía Colombiana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia
Reproduction and quotation of material appearing in the journal is authorized provided the following are explicitly indicated: journal name, author(s) name, year, volume, issue and pages of the source. The ideas and observations recorded by the authors are their own and do not necessarily represent the views and policies of the Universidad Nacional de Colombia. Mention of products or commercial firms in the journal does not constitute a recommendation or endorsement on the part of the Universidad Nacional de Colombia; furthermore, the use of such products should comply with the product label recommendations.
The Creative Commons license used by Agronomia Colombiana journal is: Attribution - NonCommercial - ShareAlike (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/.




















