Activity of pectic enzymes involved in the ripening process of lulo (Solanum quitoense Lam.)
Downloads
PHYSIOLOGY & POST-HARVEST TECHNOLOGY
Activity of pectic enzymes involved in the ripening process of lulo (Solanum quitoense Lam.)
Actividad de enzimas pécticas involucradas en la maduración del lulo (Solanum quitoense Lam.)
Jeimmy Marcela Rodríguez N.1 and Luz Patricia Restrepo S.2
1 M.Sc. student in Chemistry Sciences, Universidad Nacional de Colombia. Bogota (Colombia).
2 Chemistry Department, Sciences Faculty, Universidad Nacional de Colombia. Bogota (Colombia).
3 Corresponding autor: lprestrepos@unal.edu.co
Received for publication: 18 June, 2009. Accepted for publication: 2 February, 2011.
ABSTRACT
In the ripening process of the lulo (Solanum quitoense Lam.) physicochemical changes are produced by pectics enzymes as Polygalacturonase (PG), Pectinesterase (PE) and Pectateliase (PL) that acting on pectics substrates of plant tissue, being responsible of the physiological alteration of cells and tissues that results in the fruit softening and the beginning of the premature senescence period. This research explores the foundations of the softening enzymes behavior of lulo epicarp for the activity measurement of PL, PG and PE of fruit´s epicarp and determining their relationship with the softening process during the ripening and senescence process of fruits through follow up of the enzyme expression, the ripening index and instrumental hardness during the lulo fruit ripening under three storage treatments: 1) Control (18° C, 57 days), 2) Refrigeration (18° C, 1 day; 4° C, 14 days; 18° C, 42 days) and 3) Pre-cooling heat shock (27° C, 1 day; 4° C, 14 days; 18° C, 42 days) found that the enzymes expression and softening is reduced by heat treatment, compared with the control group; however, the cold storage inhibit the fruit softening process but chilling injuries was produced, while heat shock, in addition to inhibiting the enzymes expression, inhibited the fruit softening process and protect against chilling injuries prolonging the shelf life in 10 days, showing that it´s the best post-harvest treatment for this type of fruit.
Key words: softening enzymes, pectinesterase, polygalacturonase, pectateliase, chilling injuries, heat shock, shelf life.
RESUMEN
En el proceso de maduración del lulo (Solanum quitoense Lam.), los cambios fisicoquímicos se producen a causa de la actividad de enzimas pécticas, como la poligalacturonasa (PG), la pectinoesterasa (PE) y la pectoteliasa (PL), que actúan sobre los substratos pectínicos de los tejidos de la planta y son responsables de la alteración fisiológica de tejidos y células que derivan en ablandamiento de la fruta e inicio prematuro del periodo de senescencia. Esta investigación indaga sobre el comportamiento de las enzimas que originan el reblandecimiento del epicarpio en el fruto de lulo mediante la estimación de los niveles de PL, PE y PG; determina su relación con los procesos de maduración y senescencia mediante el seguimiento de la expresión enzimática y los índices de maduración y dureza instrumental, a través de tres tratamientos de almacenamiento: 1) Control (18° C, 57 días), 2) Refrigeración (18° C, 1 día; 4° C, 14 días; y 18° C, 42 días) y 3) Choque térmico antes del enfriamiento (27° C, 1 día; 4° C, 14 días; 18° C, 42 días), encontrándose que la expresión enzimática y el ablandamiento se reducen por el tratamiento con calor, comparado con el grupo control; sin embargo, el enfriamiento inhibe el proceso de ablandamiento pero produce daños, en tanto que el choque térmico, además de inhibir la expresión enzimática, inhibe también el proceso de ablandamiento y protege contra los daños por enfriamiento, prolongando el tiempo de conservación en 10 días; con ello se demuestra que este es el mejor tratamiento postcosecha para esta fruta.
Palabras clave: enzimas de reblandecimiento, pectinoesterasa, poligalactorunasa, pectoteliasa, daños por enfriamiento, choque de calor, tiempo de conservación.
Introduction
Lulo crops represents 1.7% of the annual production of fruits in Colombia. As a climacteric fruit, its recollected in the 40% of sensorial ripeness or "Pintón" state (Gattoni, 1994; Gómez, 1999); in the post-harvest, when the ripening process starts, changes appear due to the production of pectic enzymes that act over different substrates in the vegetal tissue, starting in the epicarpium (cortex) and then in the mesocarpium (pulp). When the fruits are not stored optimally nor transported in the correct way, changes in the pectic enzymes changes the traits of turgency, enterin the state of senescence and, as a result of this, being rejected by the final consumers (Gómez, 1999).
The researches about conservation of resources as plants and fruits are mainly of a descriptive level. The lack of information about indicators of physiological and biochemical changes in the ripening process in different conditions of storage does not allow to establish the quality and the level of perturbation of the fruits. The enzyme that has been studied have been chosen due to their part in the softening of lulo are of special interest in a biochemical level since they generate more results that allow researchers to complete the different causes of fruit deterioration, to know the degree of participation of the enzymes that take account on the softening of the fruit in the ripening process and in different ways of the fruit conservation, find when the cellular damage exceeds the overall capacity of recovery and to implement further studies that can help researchers in the modification of the molecular degradation system of the fruit support, such as pectines, in order to increase tolerance of this tropical fruit which, generally, is exposed to different environmental condition in its storage against its natural conditions.
In the fruits, pectic substances constitutes a determinant factor of the texture and the firmness, as so, the quality of the products. As a matter of fact, the characteristic softening of the fruits when mature is due to the increment if the pectinolytic enzymes that are controlled fisiologically in the plant if the fruit is not climacteric and to the post-harvest conditions of the storage when the fruit is climacteric (Ali et al., 2004; Bartley et al., 1982). The pectic enzymes that are responsible for the physiological alteration of the vegetal tissue have as a consequence the softening of the fruit and the loss of physical , chemical and sensorial traits are Pectinesterase (PE) E.C.3.1.1.11, Poligalacturonase (PG) E.C.3.2.1.67 y Pectateliase (PL) E.C.4.2.2.2, which are part of the cellular wall. The conjoint action of these enzymes in the ripening process causes the degradation of pectines and so, a better texture for the final consumer. When the enzymes are overused, an excessive enzymatic activity causes notorious softening, loss of texture and creates a perfect microconditions for a microbial attack (Pérez et al., 2000; Ali et al., 2004).
Pectinesterase (PE) is an enzyme widely found in plants and microorganisms, which hydrolyses the methylicester bods of the esterified carboxyls, releases methanol and transforms the pectin in low methoxyl pectin and even polygalacturonic acid. PE has been extracted and characterized as part of the ripening behavior of chalote (Wu et al., 2004), pear (Zhou, 2000), arracacha (Pires and Finardi-Filho, 2005), potato (Puri et al., 1982), papaya (Fayyaz et al., 1995), kiwi (Wegrzyn et al., 1992) and guava (Abu-Bark et al., 2003). It has been proposed that the control of the enzymatic action will control the process of softening, which will result in an increase of the commercialization of the fruits and a better profit and rentability of the fruits.
Polygalacturonase (PG) is the enzyme which breaks the glycosidic bonds next to a methyl-ester bond. Its action produces double-bonds between the 4 and 5 carbons of the D-galacturonic acid and, as consequence, breaks the glycosidic bond by beta-elimination mainly in high methoxyl pectins. Pectateliase (PL) has been extracted in banana (Payasi et al., 2003, 2006) and strawberries (Jiménez et al., 2002; Sesmero et al., 2007), whereas it has been proven that the diminution of PL expression in transgenic strawberries involves higher index of retention and tightness in the transformed fruits. Another study in strawberries showed that the cDNA expressed during fruit ripening are not expressed in different parts of the plant other than fruits, and so, the expression of this gene could be related to the degradation of the cell wall, contributing to the softness of the fruits.
The main objective of this research is to do a quantitative study of behavioral bases of the softening enzymes of the epicarpium of the lulo, PE, PL and PG, and to determine the relation of this enzymatic expression with the softening process and the firmness change during ripensess under three different storage conditions in order to expand the life-span of the lulo.
Materials and methods
Vegetal sampling and extraction
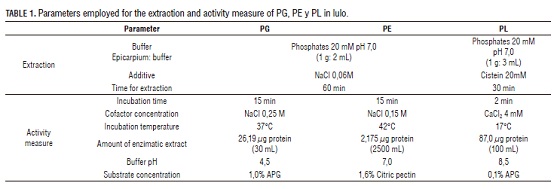
Lulos from Campohermoso, Boyaca (Colombia, 1,300 meters over the sea level) where used in this study, in maturity state I according to NTC 5093, completely green cortex without brown patches, weird odors and without defects from mechanical movement as hits or bruises. The fruits were washed and disinfected by immersion in sodium hypochlorite (0.1%), washed with abundant water and dried in a dry cloth. The epicarpium was separated from the fruit mechanically, freeing the most cortex which was macerated with liquid nitrogen until a homogenous sample was obtained. Acetone dust method was used: 1g of the homogenous sample was mixed with 6mL of acetone 4° C, shaken in vortex at 600 rpm/30s and centrifuged at 9,907 gp/10 min. The procedure was made 3 times discarding the supernatant. Once obtained the acetone dust, the pellet was re-suspended in the extraction buffer at 4° C, the extractions were made in a shaker at 180 rpm and 4° C, once finalized it was centrifuged at 9,907 gp and 4° C by 20 min, where the supernatant transformed into the crude enzymatic extract and was employed to do the activity determinations and protein quantification in each of the enzymes according to the methods in Tab. 1.
Storing assays
In order to determine the responsible enzymes of the softening in the fruits during ripening, a experimental design was used. We divided parcels in 3 different storing conditions. The first group was stored at room temperature (RT) at 18° C and 68% RH. The second group was stored on refrigeration (RS) at 4° C and 85% RH for 14 days before the complementary maturity and after it the samples were stored at 18° C and 68% RH. The third group was under thermal shock (CT) at 27° C and 37% RH for 24 hours and then stored at 4° C, 85% RH.
During storing assays a sample of each treatment was taken by triplicates the days 0, 1, 3, 6, 9, 13, 15, 17, 20, 24, 28, 34 y 57 during the ripening of the fruits. The protein of the epicarpium of each sample was quantified for the activity of PG, PE and PL as shown in Tab. 1.
Measuring the enzimatic activity
Activity of pectinesterase (PE)
The activity of PE was made under a acid-base, protonic and carboxylic group valorations after the enzymatic reaction with a titiration with sodium hydroxide 0,02M, until pH 7.0, using as substrate citric pectine 7.1 of metholxile Sigma®. A unit of PE activity (UPE) was defined as the μmols of protons generated min/mg of protein (Fayyaz et al., 1995; Zor y Sellinger, 1996).
Activity of poligalacturonase (PG)
The activity of PG was made under the spectrophotometric measure of the reducing sugars after the enzymatic action on the substrate (poligalacturonic acid, APG) at 500nm (Nelson, 1944; Somogyi, 1952; Pathak and Sanwal, 1998; Abu- Bark et al., 2003; Pires and Finardi-Filho, 2005; Prasanna et al., 2006). One unit of PG activity (UPG) was defined as nmol of reducing sugars per min/mg of protein (nKatal/s).
Activity of pectatoliase (PL)
The activity of PL was made under the formation of the products of the hydrolysis of APG quantifying the unsaturated galacturonid in the 4 and 5 carbons which absorbs at 230 nm, using e=5200 (Jiménez et al., 2002; Payasi et al., 2006). A unit of PL activity (UPL) was defined as μg of unsaturated APG min/mg of protein.
Protein quantification
Linearized Bradford's method according to Zor and Sellinger (1996) was applied, using bovine serum albumin (BSA) and reading the absorbance at 450 and 590 nm in a UV-VIS spectrophotometer Genesys™.
Physicochemical parameters evaluated during ripening
Physicochemical parameters that are affected in the process of ripening were studied. Respiratory intensity was measured by the CO2 amount (mg) produced by 1kg of fruit/h, using a CO2 sensor (Vernier Software & Technology, CO2 Gas Sensor CO2-BTA™). The tightness was evaluated by the resistance to fruit penetration using a Fruit Pressure Tester™ (FT327). The results were expressed as kg-f after 3 insertions per fruit. The ripeness index was measured as the relationship as the content of soluble solids and the titirable acidity of the fruit using a ATAGO Hand refractometer™ (NTC 4624) and citric acid percentage. Each determination was evaluated in three different fruits per treatment by triplicate.
Data analysis
Anova statistical test was used to determine the signifficative differences between days of the same treatment. Fisher's LSD tests were used to determine diference between days of each treatment. All statistical analysis were made using STAT GRAPHICSplus™ v.5.2.
Results and discussion
Response of the physicochemical parameters
The storing assays under RT (18° C, 68% RH, 57 days) where used as control, since the treatment simulated the normal ripening conditions of the fruits in Bogotá, Colombia.
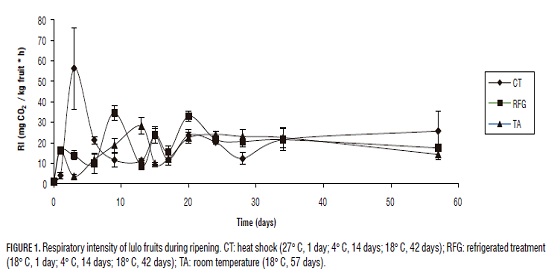
The ripening process in lulo was followed during the storing assays using respiratory intensity (RI)(Fig. 1) which permitted us to verify the normal behavior of a climacteric fruit such as processes of ripening and flavoring. The start of a climacteric ripening is a well-defined process which is characterized by a fast increase in the respiratory intensity of the fruit and the formation of ethylene by the fruit. Is also seen that the fruit are suffering stress conditions, as a change is evident in the respiration process when the fruit is submitted to a thermal treatment during CT on the third day, on RFG group on day 9, and on CT group the days 15 and 22 while in RFG is on the 20th day. Additionally, maximum respiration points were reached on the 34th day for CT against the 24th day for RFG. After having the maximum point of respiration, the fruits start senescence and start losing several traits, such as the ideal parameters of turgency, elimination of H2O due to transpiration which leads to a different aspect, the production of fermented scents (generally formed by methylic and ethylic esters of carboxylic acids and alcohols). We suggest that the thermal processes lower the respiratory activity, creates a delay in the formation of the respiratory maximum that leads to a slowering in the fruit ripening increasing its lifespan. This behavior has been also reported for araza (Eugenia stipitata Mc Vaugh) (Narváez, 2003) and "caimarona" grape (Pouroma cecropiifolia) (Narváez and Restrepo, 2003).
The behavior of the fruits during ripeness can be seen on Fig. 2. It shows that storage at RFG and CT were the best treatments on maintaining the sensorial traits of the fruits in a non-ripening state and in a good turgency state for 10 days more than TA control group. Nevertheless, RFG treatment causes damages in the epicarpium resulting from the cold storing since day 24 until the end of the treatment. In the other way, CT treatment protected the fruits from damage the same was that has been reported in Narvaéz and Restrepo (2003), Torres et.al (2003) and Sheng et al. (2006). The effect of the thermal shock on the fruit consists on the prolongation of their lifespan due to metabolism retardation, synthesis of heat shock proteins (HSP, Sheng et al. 2006), inhibition in the synthesis of proteins associated to senescence activation, inhibition in the loss of phospholipids from the cell wall that aids in the conservation of its integrity, diminution on the polyphenoloxidase activity that is responsible of the enzymatic bruising (Robards et al., 1999; Rivera et al., 2004), and the strengthening of the anti-oxidant system that prevents the accumulation of reactive species of oxygen.
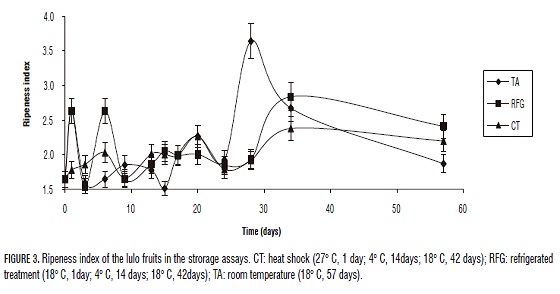
The index of maturity is the parameter that describes the best the real state of ripening of a fruit since it relates the increasing soluble solids and the titratable acidity that diminishes during the process of ripening. Its importance to notice that the characteristic sour flavor of the lulo is not loss even when the fruit has finished its process of ripening. During the process of ripening, the soluble solids rises as there is degradation of polymers of insoluble carbohydrates in molecules of simple and soluble carbohydrates and also, the titratable acidity decreases as the organic acids are converted in sugar during the respiration processes.
Fig. 3 shows the index of ripeness. It shows that the ripeness index is always greater in TA compared to the thermal treatments, suggesting that this treatments are capable of inhibit the normal process of lulo ripening, retarding the expression of the sensorial traits, which could be used as a better opportunity in the sale of a product in optimal condition. The highest index of maturity were presented in the 28th day for TA control group and on day 34 for thermal treatments RFG and CT. These results are similar to the ones produced in araza (Eugenia stipitata Mc Vaugh) and "caimarona" grape (Narváez, 2003).
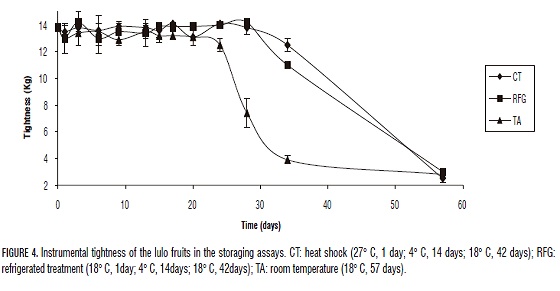
The measure of tightness in fruit ripening is the main parameter to determine the magnitude of the effect of the softening enzimes (Muchuweti et al., 2005; Payasi et al., 2006). No significant difference in tightness is shown between the different treatments until day 24, as shown in Fig. 4. These results indicate that in the first part of the treatment, the turgency is still conserved even when the enzymes responsible of the ripening process are active (Sesmero et al,. 2007). Control group (TA) presented a diminution of tightness since day 24, and increases during the next days of storage (28, 34 and 57) as a consequence of the start of the senescence period where the diminution of turgency and excessive softening are determinant traits as shown in Fig. 4 and has been reported for tomato fruits by Warrillow and Jones (1994). The group of fruits under heat shock (CT) had the same tightness that RFG until the day 28, showing that both treatments are good in the inhibition of the loss of turgency. Nevertheless, in the day 34 we found the best sensorial traits on the CT and RFG storage treatments, but when senescence starts, CT has a better tightness than RFG, suggesting that heat shock is more effective in turgency conservation, reduction of damages caused by refrigeration and has the better effectiveness in the inhibition of softness of the fruit. These results are concordant with the result found by Narváez (2003) in araza and "caimarona" grape.
Response of the enzymatic activities PE, PG and PL during maturation
During maturation, softening is existent independently from any thermal treatment, which only slows the softening process for a while. Nevertheless, the magnitude of tightness will depend on the kind of storage in which the fruits were submitted, as shown by this study and Narváez (2003). The inhibition of the softening process by means of a thermal treatment is due to the activity of the related enzymes on this process (Sheng et al., 2006).
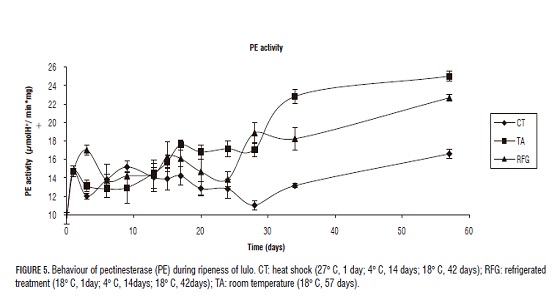
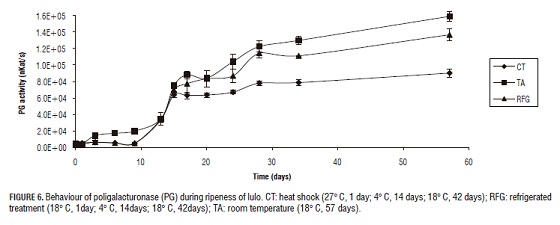
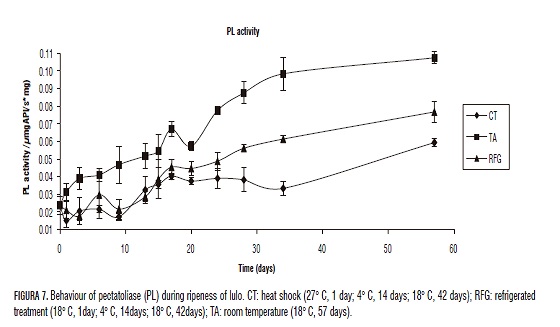
The first enzyme that intervenes in the process of softening is PE. Once PE has hydrolyzed the ester bound of the methoxylated pectin, the exo-poligalanacturase (PG) start to create the degenerative process of the poligalacturonic acid in the reductant ends hydrolyzing the glycosidic alpha-1-4 to release molecules of galacturonic acid (Muchuweti et al., 2005; Pires and Finardi-Filho, 2005; Prasanna, 2006); simultaneously the pectiolase (PL) is degrading the polygalacturonic acid by means of beta-elimination to produce molecules of insaturated galacturonic acid (Payasi et al., 2003; Payasi et al., 2006) (Figs. 5, 6 and 7).
The control group (TA) behaved as expected, where a high enzymatic activity was present in the whole time of study when compared to the other treatments. In every other treatments as the process of commercial ripening starts, the enzymatic activity rises. Heat shock effects previous to the process of refrigeration were observed for all enzymes on the complementary ripening stage (after de day 15). Figs. 5, 6 and 7 shows the significant diminution of CT against RGF and control TA suggesting that this treatment is more effective on the inhibition of PE, PG and PL and the loss of turgency, which can be extrapolated to the prolongation of the lifespan of the fruits. Thermal treatment is capable of inhibiting the activity of PE, PG and PL in the complementary ripening stage and senescence. Nevertheless, to the end of the study all three storage treatments showed the same low resistance to penetration, which shows that TC only helps the lifespan up to the day 34.
Concluding remarks
The tightness diminishes slowly in the group of fruit submitted to TC when compared to RFG and control TA. The inhibition of the process of softening is due to the inhibitory effects that RFG and CT has over the PE, PG and PL enzymes.
The excessive softening of the fruit occurs during the period of senescence of the fruit due to a considerable amount of the activities of PE, PG and PL enzymes. The process of heat shock compared to RFG and control TA inhibits the excessive softening of the fruit during senescence due to the inhibition of the activity of the PE, PG and PL enzymes.
During the process of refrigeration PE, PG and PL activities maintained latent and did not affected directly on the processes of fruit softening. Heat shock compared to RFG and control TA inhibits the excessive softening of the fruit during senescence due to the inhibition of the activity of the PE, PG and PL enzymes.
Heat shock (CT) treatment previous to the refrigeration state resulted in an increased lifespan of the fruits due to the inhibition of the activity of the softening enzymes and also protects the fruits to the damage caused by the cold, and we propose it as the best storage treatment for these kinds of products.
The present research is one of the main biochemical supports for the realization of the heat shock treatment for lulo before processes of refrigerated transport as the lifespan of the fruit increases (as shown by the green color, turgency of the fruits and a more sustainable complementary ripeness) as complementary ripeness will occur at the destination of the fruits and not in the means of transportation and commercialization.
Literature cited
Abu-Bark, A., T. Abu-Goukh and A. Bashir-Hind. 2003. Changes in pectin enzymes and cellulose activity during guava fruit ripeninig. Food Chem. 83, 213-218.
Ali, Z., L.H. Chin and H. Lazan. 2004. A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Science 167, 317-327.
Bartley, I., M. Knee, M. July 1982. The chemistry of textural changes in fruit during storage. Food Chemistry 9 (1-2), 47-58.
Fayyaz, A., B. Sabih, H. Ghazali and Y. Che-Man. 1995. Stability studies of papaya pectinesterasa. Food Chem. 53, 391-396.
Gattoni, S., 1994. El lulo: cultivo del porvenir. Carta Agraria 227-229.
Gómez, C., 1999., "Manejo integrado de cultivo de lulo"., Convenio CORPOICAPLANTE-SENA., Tecnimpresos., Ibagué Colombia., pag 85-96.
Jiménez, S., J. Redondo, J. Muñoz and J. Caballero. 2002. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 128, 751-759.
Muchuweti, M., E. Moyo and S. Mushipe. 2005. Some properties of the polygalacturonase from four zimbabwean wild fruits (Uapaca kirkiana, Zizphus mauritiana, Tamarindus indica and Berchemia discolor fruitsUapaca kirkiana, Zizphus mauritiana, Tamarindus indica and Berchemia discolor fruits). Food Chem. 90, 655-661.
Narváez, C. 2003. Efecto del choque térmico de Arazá (Eugenia stipitata Mc Vaugh) sobre la tolerancia al frío. Rev. Col. Quím. 32(2), 93-102.
Narváez, C. and L. Restrepo. 2003. Efecto del almacenamiento de uva caimarona (Pourouma cecropiifolia) a diferentes temperaturas sobre los sólidos solubles y la actividad de catalasa. Rev. Col. Quím. 32(2), 81-92.
Nelson, N. 1944. A photometric adaptation of the somogyi method for determination of glucose. J. Biol. Chem. 153, 378-380.
NTC 5093. (2002). Frutas frescas. Lulo de Castilla. Especificaciones. Fecha de ratificación 30 octubre, 2002.
NTC 4624. (1999). Jugos de frutas y hortalizas. Determinación del contenido de sólidos solubles. Método refractométrico. Fecha de ratificación 16 junio, 1999.
Pathak, N. and G. Sanwal. 1998. Multiple forms of poligalacturonase from banana fruit. Phytochem. 48, 249-255.
Payasi, A. and G. Sanwal. 2003. Pectato lyase activity during ripening of banana fruit. Phytochem. 63, 243-248.
Payasi, A., P. Misra and G. Sanwal. 2006. Purification and characterization of pectate lyase from banana (Musa acuminata) fruits. Phytochem. 67, 861-869.
Pérez, S., K. Mazeau and H. du Penhoat, C. 2000. The threedimensional structures of the pectic polisaccharides. Plant Physiology 38, 37-55.
Pires, R. and F. Finardi-Filho. 2005. Extraction and assay of pectin enzymes from Peruvian carrot (Arracacia xanthirriza B.). Food Chem. 89, 85-92.
Prasanna, V., T. Prabha and R. Tharahathan. 2006. Multiple forms of polygalacturonase from mango (Mangifera indicao l. cv Alphonso) fruit. Food. Chem. 95, 30-36.
Puri, A., T. Solomos and A. Kramer. 1982. Partial purification and characterization of potato pectinesterasa. Food Chem. 8, 203-213.
Rivera, A., L. Restrepo and C. Narváez. 2004. Polifenoloxidasa y peroxidasa de pulpa de uva caimarona (Pourouma cecropiifolia). Rev. Col. Quím. 33(1), 57-66.
Robards K., P. Prenzler, G. Tucker, P. Swatsitang and W. Glover. 1999. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 66, 401-436.
Sesmero, R., J. Quesada and J. Mercado. 2007. Antisense inhibition of pectate lyase gene expression in strawberry fruit, characteristics of fruits processed into jam. J. Food Eng. 29, 194-199.
Sheng X., L. Jianlong, Z. Xinquan, W. Hong and C. Langjun. 2006. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 56, 274-285.
Somogyi, M. 1952. Notes of sugar determination. J. Biol. Chem. 195, 19-23.
Torres R., M. Valentines, J. Usall, I. Vinnas and C. Larrigaudiere. 2003. Possible involvement of hydrogen peroxide in the development of resistance mechanisms in "Golden Delicious" apple fruit. Postharv. Biol. Technol. 27, 235-242.
Warrillow, A. and M, Jones. 1994. Different Forms Of Tomato Pectinesterase Have Different Kinetic Properties. Phytochem. 39, 277-282.
Wegrzyn, T. and E. Macrae. 1992. Pectinesterase, polygalacturonase and b-galactosidase during softening of ethylene-treated kiwifruit. Hort. Sci. 27(8), 900-902.
Wu, M., Y. Chen and J. Hwang. 2004. Transacylation of citrus pectin as catalyzed by pectinesterase from tendril shoots of chayote (Sechium edule Si). Food Res. Int. 37, 759-765.
Zhou, H. 2000. Pectinesterase, polygalacturonase and gel formation in peach pectin fractions. Phytochem. 55, 191-195.
Zor, T. and Z. Sellinger. 1996. Linearization of the bradford protein assay increases its sensitivity, theorical and experimental studies. Anal. Biochem. 236, 302-308.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2011 Agronomía Colombiana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia
Reproduction and quotation of material appearing in the journal is authorized provided the following are explicitly indicated: journal name, author(s) name, year, volume, issue and pages of the source. The ideas and observations recorded by the authors are their own and do not necessarily represent the views and policies of the Universidad Nacional de Colombia. Mention of products or commercial firms in the journal does not constitute a recommendation or endorsement on the part of the Universidad Nacional de Colombia; furthermore, the use of such products should comply with the product label recommendations.
The Creative Commons license used by Agronomia Colombiana journal is: Attribution - NonCommercial - ShareAlike (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/.