Evaluation of weeds as possible hosts of the potyviruses associated with tree tomato (Solanum betaceum Cav.) viroses
Keywords:
ELISA, titulación, tamarillo, TaLMV. (es)Downloads
To determine possible weed hosts of potyviruses associated with the disease known as “tree tomato virus disease” in Antioquia department (Colombia), a sampling was conducted to identify weed species commonly found in commercial crops of S. betaceum affected by the virus and the possible presence of the virus in these plants. The encountered weed species were grouped into seven different taxonomic families, within which we evaluated the ten most common species. The selected weeds, three indicator species of the virus and tree tomato plants were grown in a greenhouse and mechanically inoculated with an extract of infected tree tomato tissue. One month after inoculation, the tree tomato plants and Nicotiana tabacum showed symptoms of the disease and were serologically positive, whereas none of the weeds showed symptoms or were positive for potyviruses serology. In order to confirm that the detection of the virus was not caused by low viral titers that did not reach the minimum detection level of the test used, the tomato tree plants were reinoculated with an extract of sap from the studied weeds and potyviruses was not detected in any of the tested weeds and therefore cannot be considered, with the utilized methodology, as hosts for the potyviruses affecting tree tomato plants.
CROP PROTECTION
1 Grupo de Investigación Sistemas Agrícolas Tropicales. Medellin (Colombia).
2 Faculty of Agricultural Sciences, Politécnico Colombiano Jaime Isaza Cadavid. Medellin (Colombia).
3 Corresponding author. epgonzalez@elpoli.edu.co
Received for publication: 25 August, 2010. Accepted for publication: 1 March, 2012.
ABSTRACT
To determine possible weed hosts of potyviruses associated with the disease known as "tree tomato virus disease" in Antioquia department (Colombia), a sampling was conducted to identify weed species commonly found in commercial crops of S. betaceum affected by the virus and the possible presence of the virus in these plants. The encountered weed species were grouped into seven different taxonomic families, within which we evaluated the ten most common species. The selected weeds, three indicator species of the virus and tree tomato plants were grown in a greenhouse and mechanically inoculated with an extract of infected tree tomato tissue. One month after inoculation, the tree tomato plants and Nicotiana tabacum showed symptoms of the disease and were serologically positive, whereas none of the weeds showed symptoms or were positive for potyviruses serology. In order to confirm that the detection of the virus was not caused by low viral titers that did not reach the minimum detection level of the test used, the tomato tree plants were reinoculated with an extract of sap from the studied weeds and potyviruses was not detected in any of the tested weeds and therefore cannot be considered, with the utilized methodology, as hosts for the potyviruses affecting tree tomato plants.
Key words: ELISA, titration, tamarillo, TaLMV.
RESUMEN
Para determinar las posibles arvenses hospedantes de potyvirus asociados a la enfermedad conocida como "virosis del tomate de árbol" en el departamento de Antioquia (Colombia), se llevó a cabo un muestreo para identificar las especies arvenses más comúnmente encontradas en cultivos comerciales de S. betaceum afectados por la virosis y la posible presencia del virus en estas plantas. Las especies de arvenses encontradas fueron agrupadas en siete familias taxonómicas diferentes, dentro de las cuales se evaluaron las diez especies más frecuentes. Las arvenses seleccionadas, tres especies indicadoras de virus y tomate de árbol, fueron cultivadas en invernadero e inoculadas mecánicamente con extracto de tejido de tomate de árbol infectado. Un mes después de la inoculación, tomate de árbol y Nicotiana tabacum, mostraron síntomas dela enfermedad y fueron positivas en la prueba serológica, mientras que ninguna de las arvenses mostró síntomas ni fueron positivas en la serología para potyvirus. Con el fin de corroborar que la no detección del virus fuese ocasionada por bajos títulos virales que no llegaban nivel mínimo de detección de la prueba utilizada, se re-inocularon plantas de tomate de árbol con extracto de savia de las arvenses en estudio, encontrándose que no fue detectado potyvirus en ninguna de las arvenses evaluadas por lo que no pueden ser consideradas mediante la metodología utilizada como hospedantes de potyvirus que afectan las plantas de tomate de árbol.
Palabras clave: ELISA, titulación, tamarillo, TaLMV.
Introduction
The tree tomato (Solanum betaceum Cav.) has gained great economic importance in Colombia because it is a highly nutritious fruit and its organoleptic characteristics make it ideal for industrial processing and entry into international markets. Antioquia is the largest producer of the fruit in the country with a planted area of 1867.3 ha and production of 57,105.9 t. This state also reported higher yields of about 30 t ha-1, compared to the national average yields that remain around 18 t ha-1 (MADR, 2006).
Among the various pathogens affecting tree tomato crops, viruses stand out due to their obvious economic impact on commercial orchards, not only in the state and country but also the world (Cruz, 2005). Although the damage has not been accurately quantified, Tamayo (1996) reported losses of about 50% due to viruses. This disease is mainly caused by potyviruses, recently named Tamarillo leaf malforma- tion virus (TaLMV) (Ayala et al., 2010), which also causes a significant reduction in the planted area, reducing the productive period of the fruit tree, manifested both in Antioquia and the rest of the country (Gil et al., 2009; Ayala et al., 2010). Subsequent studies reported the presence of the isometric viruses AMV, CMV and PLRV in Antioquia with the ELISA test, expanding reported viruses that can affect the tree tomato (Jaramillo et al., 2011).
Symptoms of diseased S. betaceum plants are characterized by mosaic, chlorosis, deformation of leaf floral and fruit tissue. Depending on environmental conditions, symptoms may include deformation and elongation of leaves, mosaic, mottle, thickening of the veins and presence of blisters or bladders on the leaf lamina. Symptoms on the fruit appear as circular and rectangular spots of varying shades of purple that may cover part or all of the fruit; the color can change to reddish tones as they mature (Jaramillo, 2011). Occasionally some affected fruits are deformed and the flesh becomes dry and acidic (Bernal and Díaz, 2003). Gil et al. (2009) observed in young plants mechanically inoculated with diseased plant extract symptoms such as leaf chlorosis in new growth, mosaic, curling toward the back, blisters, poor lanceolated leaves and poor foliage as compared to non-inoculated controls.
Potyviruses transmission can occur mechanically through tools, by insect vectors associated with the crop, especially aphids in a non-persistent manner (Martínez et al., 2010) or even by seed (Díaz et al., 2010; Álvarez et al., 2011).
In studies on virus plant reservoirs, especially indicators, Saldarriaga et al. (1998), assessed virus transmission in the tree tomato and found that of 22 indicator species, only five, Nicotiana bentamiana, Nicotiana rustica, Chenopo- dium murale, Datura metely and Solanum nigrum had symptoms associated with the disease, with only C. murale and S. nigrum positive for the Col 7 antiserum, which was produced at a particle isometric, and negative for Col 11, and in turn, produced an elongated flexuosa particle. The latter was reported as presumptive for potyviruses. The other species were not positive for any of the antisera. In the same study, N. tabacum had no symptoms and was not positive for any of the antibodies, which contrasted with the case of N. glutinosa, which was positive for the antibody Col 7 but had no symptoms.
Weeds play an important role as a virus reservoir, vector or both at once. Apablaza et al. (2003) found in studies on weeds surrounding economically important crops with viral diseases and their role in the spread of these diseases that the density of the weeds is an important part in the development of viral vectors associated with the crops of interest and the weeds, as in determining the host range of the Beet western yellows virus (BWYV, Polerovirus) in beets and the Potato leaf roll virus (PLRV, Polerovirus) in the potato in Canada, where more than 10,000 plants were sampled using serological tests, it was found that only 12 species belonging to five families positive for BWYV were positive for the virus, four of which had not been reported as hosts of the pathogen (Ellis, 1992).
This study was developed based on a search for weed species that are present in greater proportion in cultivated tree tomatoes, with the premise that these plants could be involved in the quick spread of tree tomato viruses in Antioquia, especially in newly cultivated lots. We studied the ten most common weeds in tree tomato crops and their relationship with the tree tomato virus with inoculation tests, symptom monitoring and a specific serological test for the genus Potyvirus.
Materials and methods
Identification of the most common weeds in tree tomato crops
Identification was carried out on two commercial tree tomato lots located in the municipalities of Entrerrios and San Pedro de los Milagros (Antioquia), one three months after transplant and the other established for two years. The square method was used for weed recognition in the tree tomato crop, which consisted of a 1 x 1 m frame placed at random in the lot to allow randomization of sampling. Sampling was done a hundred times on each hectare to cover an area corresponding to 1%. In each quadrant, all encountered species were collected and subsequently identified by their morphological characteristics, selecting the ten most common species in both tree tomato lots. Taxonomic characterization was performed with the help of the Herbarium at the Universidad Nacional de Colombia, Medellin.
Weed propagation, indicator plants and the tree tomato
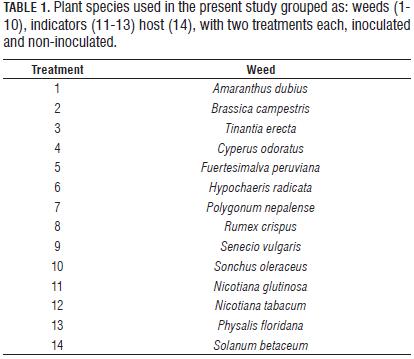
To determine the range of host species, this study evaluated the ten weed species most frequently found in the plots (Tab. 1) and three species of indicator plants for Potyvirus.
Propagation of the ten weed species and the three indicator species was sexual, by seed, in the greenhouse in the Secretaria de Agricultura de Antioquia nursery, Niquía- Bello (Antioquia).
Propagation of the tree tomato plants was also sexual, by seed, in a separate greenhouse from the weeds, protected by a small mesh house to prevent infection during the study. At three months of age, twelve plants were selected and inoculated, which were placed in an anti-aphid, mesh house with the dimensions of 1 m high and 2 m length, in the Corpoica research center "La Selva" Rionegro (located at a height of 2,150 m a.s.l. with a temperature of 17°C and annual rainfall of 1,900 mm).
From among the weeds and indicator plants that were about two months old, the youngest, most vigorous and uniform were selected of which twelve plants per weed species and five plants per indicator species were taken and moved to greenhouses at Corpoica "La Selva" Rionegro and placed in an anti-aphid, mesh house. The plants were inoculated at two months due to the short cycles of some of the weeds that did not permit inoculation at three months. Virus identification
In the municipality of San Pedro de los Milagros, Tesorero District, in a lot of adult tree tomato plants affected by symptoms associated with potyviruses, tissue samples were taken from plants affected by the disease; mainly leaves, flowers and fruits. These samples were sent to the Laboratory of Virology of the Centro Internacional de Agricultura Tropical (International Center for Tropical Agriculture - CIAT) for viral particle recognition by electron microscopy transfer. Serological detection was performed using the commercial kit specific for the Potyvirus group (Agdia, Elkhart, IN), which is based on the ELISA test (PSA 27200/0288), with antibody detection and a conjugate, both monoclonal. The latter is associated with alkaline phosphatase to reveal P-nitrophenyl phosphate (PNP) as the substrate for the enzymatic reaction. The positive and negative data were established by qualitative criterion, with positive being those reactions where there was yellow in the well with the substrate and was subdued. Negative reactions were observed where the substrate was transparent after 45 min of incubation, given that the observation indications last a maximum of 30 min after the test. With the kit, a test was done on material collected from tomato tree plants, which showed a positive result and was taken as inoculum for potyviruses multiplication in twenty S. betaceum plants at three months of age and were maintained as a reservoir and used for subsequent inoculation of selected weeds, indicators and tree tomato plants.
Mechanical inoculation of weeds, indicator plants and tree tomato plants
After a week of adaptation for the plants in the anti-aphid, mesh house, three groups of plants (weeds, indicator plants and tree tomato plants) were selected for inoculation. Eight plants were taken for each weed species, eight for the tree tomato and three plants per indicator species. Noninoculated plants were used as the control, leaving four plants per weed species, four of the tree tomato and two per indicator species. Virus inoculation was done using the methodology described below.
As a source of inoculum tree tomato plant leaves were used which were previously infected with the virus and symptomatics (plants reservoir). For every gram of leaf macerate, 10 mL of distilled water was added (ratio 1:10), the receptacle in which the mash was prepared was cooled (expanded polystyrene cuvette with ice) and with the help of gauze the tissue was rubbed with the intention of causing a wound to inoculate it (Mejía et al., 2009). Inoculation of the studied plants was performed in fully developed young leaves, near the apex, to observe systemic infection by the virus in the new shoots.
Treatments
Treatments were divided into three groups; the first corresponded to the ten weed species studied. Weed treatments consisted of eight inoculated plants and four non-inoculated. The second group consisted of three indicator plant species, in which, due to the little material which was available, three were inoculated for each species, leaving two non-inoculated controls. In the third group, there were 12 tree tomato plants of which eight were inoculated and four non-inoculated. Thus, in total, 14 species were used for a total of 28 treatments (14 inoculated and 14 noninoculated). The three groups of plants used in this study are described in Tab. 1.
The eight inoculated S. betaceum plants were used as a virus control to observe the appearance of the symptoms and verify the effectiveness of the inoculation.
Re-inoculation in the tree tomato (S. betaceum)
A lot of fifty tree tomato plants was propagated sexually, by seed. At three months of age, the thirty best plants were selected to be inoculated with macerated weeds of the present study. Each weed species had 10 g of leaf taken which was macerated following the same methodology mentioned above for the mechanical inoculation of the plant material. Three tree tomato plants were inoculated for each kind of weed for evaluation, for a total of thirty plants, which were located in the anti-aphid mesh house again. As a negative control, three plants were inoculated with distilled water.
Evaluation of weeds, indicator plants and tree tomato plants
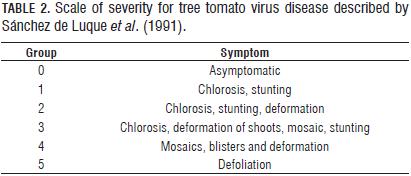
Eight days after the virus inoculation, assessments of symptoms began at periods of eight days over a month in all plants, employing the scale described by Sánchez de Luque et al. (1991) seen in Tab. 2. The evaluation was also carried out on plants re-inoculated with sap from weeds for the same period as the others (one month). At fifteen days, after the inoculation, the specific ELISA Potyvirus test was used taking fractions of each leaf sample to form 100 mg of leaf tissue which was macerated with extraction buffer 1X per manufacturer's instructions in order to confirm the presence or absence of the virus and its identity in the studied materials.
Results and discussion
The collection of weeds in the field oscillated between thirty to fifty species. Of all the species found in the field, the following were identified: A. dubios, S. oleraceus, Sonchus asper, B. campestris, T. erecta, C. odoratus, F. peruviana, P. nepalense, H. radicata, Taraxacum officinale, S. vulgaris, R. crispus and Rumex obtusifolius.
After the square method sample counts, we selected the ten most common weed species in the crop, which were the following: A. dubios, S. oleraceus, B. campestris, T. erecta, C. odoratus, F. peruviana, P. nepalense, H. radicata S. vulgaris and R. crispus.
In mechanical inoculation, the tree tomato and indicator plants began to show symptoms two weeks after inoculation with sap from diseased plants. At that time, there was slight chlorosis in the new growth of N. tabacum inoculada. In turn, the inoculated tree tomato plants showed slight chlorosis between veins and mosaic, clearly visible in the new shoots. The remaining plants did not show any symptoms associated with viruses.
At 23 d after inoculation, six of eight tree tomato plants showed symptoms associated with viruses, such as mosaic and distortion of new growth as seen in Fig. 1. N. tabacum presented a strong mosaic accompanied by a slight deformation of the leaf lamina. Other indicator plants and weeds showed no symptoms at all.

One month after mechanical inoculation of the virus, the weed species showed no symptoms associated with viruses compared with that seen in N. tabacum and the tree tomato. Wherein, as noted above, the characteristic virus symptoms presented as mosaic, chlorosis towards the base of the leaves with crinkling and stunted development as compared to non-inoculated plants.
All tree tomato plants showed symptoms of the viruses, indicating inoculation efficiency of 100%, higher than that reported by Chávez and Varón (2003) and Betancourth et al. (2003) who reported efficiencies of 17 and 80% respectively. The symptoms found in the tree tomato presented the same as in the inoculum reservoir plants, confirming that the same viral agent was transmitted, which was confirmed as potyviruses in all cases with ELISA.
N. tabacum and N. glutinosa plants have been reported as diagnostic indicators of Tamarillo mosaic virus (TaMV, Potyvirus) (Kaminska et al., 2006). Sánchez de Luque (1982) reported that an efficient indicator for the tree tomato virus was found in Cundinimarca, however in this study only N. tabacum was efficient as an indicator, while N. glutinosa and P. floridana did not show any symptoms.
Fifteen days after inoculation, the ELISA test specific for Potyvirus was used on all the study material (weeds, indicator plants and tree tomato), for both inoculated and non-inoculated. The results showed that the inoculated tree tomato plants and N. tabacum were positive, while all the other plants were not. This result may be due to synergism seen in the weeds for the expression of symptoms and virus replication, which could lead to non-detection by ELISA.
In the re-inoculation test, for each weed species, three tree tomato plants were inoculated to rule out the possibility that the studied weeds were asymptomatic carriers of the virus or other viruses associated with the tree tomato.
Evaluations were performed every week for a month, during which time no symptoms associated with the disease were verified in plants and there were no positive ELISA tests, which confirmed that by this method these species are not carriers for the Potyvirus associated with the viruses of the tree tomato in Antioquia.
In a recent study, Ayala et al. (2010) described more than 15 symptoms associated with the disease and at least two potyviruses present in the samples tested by molecular methods. One of these was the Potato virus Y (PVY) and the second a tentative species called Tamarillo leaf malforma- tion virus (TaLMV). In the same study, the incidence of the virus was evaluated with ELISA with universal antibodies for the genus Potyvirus. In the different municipalities of Antioquia, where the sampling was done, the incidence values ranged between 80 and 93%. In 2007, a rate of 2.7 was reported in lost and eradicated crops in relation to new cultivated area. This indicates that there was a net decrease in the area planted with this crop in the department (Gobernación de Antioquia, 2008), which in turn is consistent with virus incidence data of diseases reported by Ayala et al. (2010).
The world has reported joint occurrence of different viruses affecting the tree tomato, prioritizing the incidence of Potyvirus. In Ecuador, for example, the predominant virus is PVY, while in New Zealand, incidences of up to 100% of Tamarillo mosaic virus (TaMV) have been reported. Additionally, it has been frequently detected in the presence of other viruses such as: PLRV, Tomato rings pot virus (ToRSV, Nepovirus) and Alfalfa mosaic virus (AMV, Alfamovirus) in the valleys of the Pichincha region of Ecuador, while Cucumber mosaic virus (CMV, Cucumovirus), Potato au- cuba mosaic virus (PAMV, Potexvirus), Alfalfa mosaic virus (AMV, Alfamovirus), Tomato spotted wilt virus (TSWV, Tospovirus) and Arabis mosaic virus (ArMV, Fabavirus) have been diagnosed in crops in New Zealand (Vizuete et al., 1990; Eagles, 1994; Eagles et al., 1994).
Jaramillo (2011) recently reported, with the use of serological tests, the presence of at least seven viruses associated with disease in tree tomato crops in the country, with potyviruses and CMV as the most frequently encountered.
All current information evidences the need for further studies on the range of field hosts of the virus associated with the disease, especially potyviruses, as this seems to be the viral group that plays a major role; and to gain knowledge of potyviruses vectors, preferably analyzing insects associated with the crop, addressing weeds of high and low frequencies.
However, one must not forget that the seed may be another common factor in the spread of the disease, since as mentioned Gibbs and MacKenzie (1997), and Álvarez et al. (2011), Potyvirus and especially TaLMV can eventually be transmitted this way.
For the evaluation of weeds, a more sensitive test for detection may be required, such as molecular tests (molecular hybridization or specific RT-PCR), as it is feasible that low viral titers do not permit detection in the weeds by serology and re-inoculation, but they were not used in the present study because it was a primary, exploratory study which looked for field serological detection tools with inoculum sources in tree tomato plants.
Conclusions
This study presented no weed carriers of potyviruses associated with tree tomato viruses in either the inoculation trials or through Indirect-ELISA serological test. The incubation period of the present virus or viruses is 15 days in both S. betaceum and N. Tabacum and plants which corresponds to at least one virus of the Potyvirus genus. The N. tabacum species is noteworthy as an efficient indicator for the diagnosis of potyviruses associated with tree tomato viruses. New epidemiological studies are recommended to provide better information on the dissemination mechanisms of potyviruses in this crop, to improve control strategies.
Acknowledgements
This study was developed with financial support from the Dirección de Investigación y Posgrados, Facultad de Ciencias Agrarias at the Politécnico Colombiano Jaime Isaza Cadavid, the Grupo de Investigación en Mejoramiento de Frutales Andinos y Tropicales at the Universidad Nacional de Colombia, Medellin and Corpoica "La Selva".
Literature cited
Álvarez, J., J.M. Cotes, and M.M. Marín. 2011. Detección de virus asociados al material de siembra de tomate de árbol en Colombia. Biotecnología en el Sector Agropecuario y Agroindustrial 9(1), 43-50.
Apablaza, G., J. Apablaza, P. Reyes, and E. Moya. 2003. Determinación de virosis e insectos vectores en malezas aledañas a cultivos hortícolas. Cien. Inv. Agr. 30(3), 175-186.
Ayala, M., P. González, P. Gutierrez, J. Cotes, and M. Marín. 2010. Caracterización serológica y molecular de potyvirus asociados a la virosis del tomate de árbol en Antioquia (Colombia). Acta Biol. Colomb. 15(3), 143-162.
Bernal E., A.J. and C.A. Díaz D. 2003. Tecnología para el cultivo del tomate de árbol. Technical Manual No. 3. Research Center "La Selva", Corpoica, Rionegro, Colombia.
Betancourth, C., R. Goye, and D.A. Bravo. 2003. Caracterización biológica de un virus tomate de árbol (Solanum betaceum Send) en el departamento de Nariño. Fitopatol. Colomb. 27(1), 7- 10.
Chávez, B. and F. Varón de A. 2003. Enfermedad de etiología viral en cultivos de tomate de árbol. Bol. Epidemiología Agrícola ICA, Bogota. pp. 39-43.
Cruz, L.F. 2005. Identificación de virus en Solanum betaceum. Undergraduate thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota.
Díaz, A., M. Quiñones, F. Arana, M. Soto, and A. Hernández. 2010. Potyvirus: características generales, situación de su diagnóstico y determinación de supresencia en el cultivo del pimiento en cuba. Rev. Protección Veg. 25(2), 69-79.
Eagles, R.M. 1994. Tamarillo mosaic potyvirus: characterization and resistance. Ph.D. thesis. University of Auckland, Auckland, New Zealand.
Eagles, R.M., R.C. Gardner, and R.L.S. Forster. 1994. Incidence and distribution off six viruses infecting tamarillo (Cyphooomandra betacea) in New Zealand. N.Z. J. Crop Hort. Sci. 22, 453-458.
Ellis, P.J. 1992. Weed hosts of beet western yellows virus and potato leafroll virus in British Columbia. Plant Dis. 76(11), 1137-1139.
Gibbs, A. and A. Mackenzie. 1997. A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. J. Virol. Methods 63(1-2), 9-16.
Gil, J.F., M.L. Ayala, M. Marín, and E.P. González. 2009. Identificación de Potyvirus en cultivos de tomate de árbol (Solanum betaceum Cav.) en Antioquia mediante detección serológica. Rev. Politéc. 8, 112-120.
Gobernación de Antioquia. 2008. Anuario estadístico agropecuario 2007: Estadísticas Agropecuarias por Concenso. In: www.antioquia.gov.co; consulted: March, 2012.
Jaramillo, M., P. Gutiérrez , J. Cotes, P. González, and M. Marín. 2011. Detección de los virus AMV, CMV y PLRV en cultivos de tomate de árbol (Solanum betaceum Cav.) en Antioquia, Colombia. Rev. Fac. Nal. Agron. Medellin 64(1), 5831-5844.
Kaminska, M., T. Malinowski, A. Rudzinska-Langwald, and L.C. Díaz. 2006. The occurrence of Wisteria vein mosaic virus in Wisteria floribunda DC plants in Poland. J. Phytopathol. 154(4), 414-417.
MADR, Ministerio de Agricultura y Desarrollo Rural. 2006. Observatorio agrocadenas Colombia. In: http://www.agrocadenas.gov.co; consulted: March, 2012.
Martínez, J.E., J.M. Cotes, and M.M. Marín. 2010. Detección serológica y molecular de virus en áfidos asociados a cultivos de tomate de árbol con síntomas de virosis en Antioquia y Nariño (Colombia). Rev. Fac. Cienc. Básicas 6(2), 182-197.
Mejía, A.D.M., G.E.I. Rodas, H.L. Patiño, and J.E.P. González. 2009. Efecto del acibenzolar-s-metil sobre el desarrollo de la virosis causada por Potyvirus en tomate de árbol. Agron. Colomb. 27(1), 87-93.
Saldarriaga, A.C., J.L. Zapata, and J.E. Bernal. 1998. Estudios de evaluación de la transmisión del virus del tomate de árbol. Corpoica, Medellín, Colombia.
Sánchez de Luque, C. 1982. Estudios de hospedantes de un nuevo virus en el tomate de árbol (Cyphomandra betacea Sendt). pp. 41-42. In: Resúmenes V Congreso Asociación Colombiana de Fitopatologia and XXII Reunión Anual de la Sociedad Americana de Fitopatologia. Ascolfi, División Caribe, ASP, Cali, Colombia.
Sánchez de Luque C., C. de la Rota, and A. Suarez. 1991. Posibles virus en tomate de árbol (Cyphomandra betacea Sendt). Actualidades ICA 5(58), 10.
Tamayo, P.J. 1996. Enfermedades virales del tomate de árbol (Cy- phomandra betacea (Cav). Sendt.) en Colombia. ASCOLFI Informa 22, 26-29.
Vizuete, B., M.L. Insuasti, J. Ochoa, and M. Ellis. 1990. Biological and serological characterization of tree tomato virus diseases in Ecuador. In: Ohio State University, http://www.oired.vt.edu/ipmcrsp/Publications/AnnualReports/2002/Ecuador/ecuador_topic11.pdf; consulted: March, 2012.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2012 Agronomía Colombiana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia
Reproduction and quotation of material appearing in the journal is authorized provided the following are explicitly indicated: journal name, author(s) name, year, volume, issue and pages of the source. The ideas and observations recorded by the authors are their own and do not necessarily represent the views and policies of the Universidad Nacional de Colombia. Mention of products or commercial firms in the journal does not constitute a recommendation or endorsement on the part of the Universidad Nacional de Colombia; furthermore, the use of such products should comply with the product label recommendations.
The Creative Commons license used by Agronomia Colombiana journal is: Attribution - NonCommercial - ShareAlike (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/.




















