Polyphenols distributions and reserve substances analysis in cacao somatic embryogenesis
Análisis de distribución de polifenoles y sustancias de reserva en embriogénesis somática de cacao
DOI:
https://doi.org/10.15446/abc.v21n2.50196Palabras clave:
antioxidants, histology, reserve accumulation, recalcitrance, somatic embryogenesis, acumulación de reservas, antioxidantes, embriogénesis somática, histología, recalcitrancia. (en)acumulación de reservas, antioxidantes, embriogénesis somática, histología, recalcitrancia (es)
Descargas
In order to understand the causes of lack of regeneration in cacao somatic embryos, two cacao varieties with different responses to regeneration potential were described based on their capacity to store different compounds. It is well known that seed reserves play a central role in the regenerative capability of somatic embryos; thus, we followed histochemical changes and reserve fluctuations of proteins, polysaccharides and polyphenols during somatic embryogenesis (SE) in the two cacao varieties. The study showed that, in somatic embryos of the regenerating variety, polyphenols were localized mainly in the periphery of the embryo (epidermal cells) and proteins were the main storage substance in the embryo expression medium, while the non-regenerating variety had a high presence of polysaccharides with random distribution of polyphenols at the end of the embryo induction step.
Dos variedades de cacao con diferentes respuestas a la regeneración fueron descritas en función de su capacidad para almacenar diferentes compuestos, con el fin de aproximarse al entendimiento de las causas de la falta de regeneración en embriones somáticos de cacao. Es bien sabido que las reservas de semillas desempeñan un papel central en la capacidad de regeneración de embriones somáticos; por tanto, se realizó un seguimiento de cambios histoquímicos y fluctuaciones de reserva de proteínas, polisacáridos y polifenoles durante la embriogénesis somática (SE) en dos variedades de cacao. El estudio mostró que, en los embriones somáticos de la variedad regenerante, los polifenoles se localizaron principalmente en la periferia del embrión (células de la epidermis) y las proteínas fueron el componente principal de almacenamiento en el medio de expresión de embriones, mientras que la variedad no regenerante tenía una alta presencia de polisacáridos y una distribución aleatoria de los polifenoles en el final de la etapa de inducción de embriones.
Doi: https://doi.org/10.15446/abc.v21n2.50196
POLYPHENOLS DISTRIBUTION AND RESERVE SUBSTANCES ANALYSIS IN COCOA SOMATIC EMBRYOGENESIS
Análisis de distribución de polifenoles y sustancias de reserva en embriogénesis somática de cacao
Adriana María GALLEGO RÚA1, Ana María HENAO RAMÍREZ1, Aura Inés URREA TRUJILLO1, Lucía ATEHORTÚA GARCÉS1.
1 Universidad de Antioquia, Sede de Investigación Universitaria SIU. Calle 62 nº. 52-59. Medellín, Colombia.
For correspondence. adriana.gallego.02@gmail.com
Received: 15th April 2015, Returned for revision: 14th August 2015, Accepted: 8th September 2015. Associate Editor: Xavier Marquínez Casas.
Citation / Citar este artículo como: Gallego Rúa AM, Henao Ramírez AM, Urrea Trujillo AI, Atehortúa Garcés L. Polyphenols distributions and reserve substances analysis in cacao somatic embryogenesis. Acta biol. Colomb. 2016;21(2):335-345. doi: https://doi.org/10.15446/abc.v21n2.50196
ABSTRACT
In order to understand the causes of lack of regeneration in cacao somatic embryos, two cacao varieties with different responses to regeneration potential were described based on their capacity to store different compounds. It is well known that seed reserves play a central role in the regenerative capability of somatic embryos; thus, we followed histochemical changes and reserve fluctuations of proteins, polysaccharides and polyphenols during somatic embryogenesis (SE) in the two cacao varieties. The study showed that, in somatic embryos of the regenerating variety, polyphenols were localized mainly in the periphery of the embryo (epidermal cells) and proteins were the main storage substance in the embryo expression medium, while the non-regenerating variety had a high presence of polysaccharides with random distribution of polyphenols at the end of the embryo induction step.
Keywords: antioxidants, histology, reserve accumulation, recalcitrance, somatic embryogenesis.
RESUMEN
Dos variedades de cacao con diferentes respuestas a la regeneración fueron descritas en función de su capacidad para almacenar diferentes compuestos, con el fin de aproximarse al entendimiento de las causas de la falta de regeneración en embriones somáticos de cacao. Es bien sabido que las reservas de semillas desempeñan un papel central en la capacidad de regeneración de embriones somáticos; por tanto, se realizó un seguimiento de cambios histoquímicos y fluctuaciones de reserva de proteínas, polisacáridos y polifenoles durante la embriogénesis somática (SE) en dos variedades de cacao. El estudio mostró que, en los embriones somáticos de la variedad regenerante, los polifenoles se localizaron principalmente en la periferia del embrión (células de la epidermis) y las proteínas fueron el componente principal de almacenamiento en el medio de expresión de embriones, mientras que la variedad no regenerante tenía una alta presencia de polisacáridos y una distribución aleatoria de los polifenoles en el final de la etapa de inducción de embriones.
Palabras clave: acumulación de reservas, antioxidantes, embriogénesis somática, histología, recalcitrancia.
INTRODUCTION
Theobroma cacao L is a woody plant cultivated as an important industrial crop throughout tropical regions worldwide. In the last decade, its consumption has increased due to the functional properties conferred by its antioxidant polyphenols. Cacao is well known for its genetic variability due to its natural propagation system (allogamous), which generates a high degree of yield variation (Maximova et al., 2002). Seeds are one of the main sources of genetic variation in cacao because they are, in most cases, the result of cross linking between two genotypes. To minimize the negative effects of seed propagation, traditionally the crops are propagated by asexual propagation, such as rootstocks or grafting. However, these techniques have been described as inefficient and costly (Figueira and Janick, 1995). A potential solution to these problems could be found using selected tissue culture techniques. In cacao, somatic embryogenesis is the most frequently adopted regeneration method, which has been used not only for plant propagation but also for genetic engineering (Loyola and Vasquez, 2006), virus eradication (Quainoo and Dwomon, 2012a) and germplasm preservation (Fang et al., 2004; Juarez, 2012).
Although somatic embryogenesis has been reported for several cacao varieties from different explants including zygotic embryos, leaves, nucellus and floral explants (Sondahl et al., 1993; Figueira and Janick, 1995; Li et al., 1998; López-Baez et al., 2001; Maximova et al., 2002), a low conversion rate of somatic embryos into normal plants has remained a problem, limiting the utilization of embryogenic cells for genetic transformation and plant breeding (Suarez and Bozhkov, 2008). This in vitro recalcitrance in cacao is not universal, as there are both recalcitrant and non-recalcitrant genotypes. Recalcitrance can be defined as the inability of plant tissue cultures to respond to in vitro manipulations. In broadest terms, tissue culture recalcitrance also concerns the time-related decline and/or loss of morphogenetic competence and totipotent capacity (Benson, 2000). Understanding the causes of recalcitrance in cacao somatic embryogenesis would allow for the determination of in vitro morphological patterns that indicate which genotypes will regenerate during the earlier in vitro process.
Histological analyses have demonstrated deficiencies in reserve substances in somatic embryos compared with their zygotic homologues (Alemanno et al., 1997; Dodeman et al., 1997), which explains the lack of regeneration in the former. In this respect, information related to nutrient components in the endosperm has also been studied (Dangou et al., 2007). Many perennial plants, such as cacao, are notorious for their ability to produce secondary products, many of which may be phenolic in character. Polyphenol presence has also been associated with in vitro recalcitrance (Bailey-Serres and Mittler, 2006). No histological reports regarding cell features (such as polyphenols distribution) currently exist, and it remains to be identified what causes the lack of regeneration in cacao varieties exposed to tissue culture. The information derived from the cacao embryogeny in non-regenerating and regenerating varieties in tissue culture would allow for recognition of the stages of vigor and subsequent germination (Merkle et al., 1995). In this paper, we evaluate the differences in the distribution of reserve substance level and polyphenols between two varieties, one non-regenerating (ICS95) and one regenerating (BIOB, Colombian variety) in order to understand possible causes of lack regeneration in cacao somatic embryos.
MATERIALS AND METHODS
Explant material
Tissue culture experiments were conducted with two cacao genotypes from San Vicente de Chucuri, Santander, Colombia, coded as BIOB and ICS95. Immature flowers were stored in sterile basal DKW salts (Driver and Kuniyuki, 1984) on ice during transportation and carried to the laboratory for the experiment. Flower bottoms were disinfected according to the protocol of Urrea et al. (2011).
In previous research (Urrea et al., 2011), we evaluated two types of explants (staminode and petals) and two media culture protocols (Fontanel et al., 2002; Guiltinan et al., 2003) for both varieties BIOB and ICS95. Based on these previous results, we selected the treatments in which embryo formation (present in BIOB and ICS95) and regeneration (present only in BIOB) were present.
Culture procedure
Primary somatic embryos from cacao staminodes and petals were produced from two varieties, as previously described (Urrea et al., 2011). The used protocols were divided into three steps: calli induction, embryo expression and maturation, each one composed by a different media.
Between 50 and 100 explants were cultured for each variety. The cultures were incubated at 25 °C in dark and light conditions according to the protocols, and they were subcultured at four-week intervals in fresh media.
Light microscopy
Floral explants (staminodes and petals), calli and embryogenic calli were prepared for conventional light microscopy according to a method modified from Maximova et al. (2002). In brief, a time-course experiment was performed in which embryogenic and non-embryogenic samples (n=56) were fixed for histological examination at 0, 7, 15, 30, 45, 60 and 72 days after culture establishment. The samples were fixed in paraformaldehyde 2% (w/v), glutaraldehyde 1 % (v/v) and caffeine 1 % (w/v) in phosphate buffer (0.2 M, pH 7.2) and then dehydrated in ascending ethanol series (v/v): 70, 80, 90, 95 and 100 % for 20 minutes at each step, followed by imbibition in PARAPLAST paraffin wax (Mercedes Medical, Sarasota, Florida, USA) at 60 ºC. The specimens were cut into blocks and sectioned at 3 µm using a rotary microtome (LEICA Model RM 2125). Sections were double-stained with periodic acid-Schiff reactions (PAS, Merck HX106073) combined with Naphthol Blue-Black (Sigma, USA, 5374263). Samples were in acid periodically for 5 min and then rinsed three times with running tap water followed by distillated water. After, they were submerged in Schiff's reagent for 15 min in the dark. The slides were then rinsed again with running tap water and distilled water (three times).
Subsequently, slides were stained with Naphthol Blue Black (1 g Naphthol Blue Black in 100 ml 7 % [v/v] acetic acid) for five minutes followed by rinsing under running tap water plus acetic acid 10 % and heating at 60 ºC for 15 min. PAS stains starch reserves and cell wall pink (Martoja and Martoja, 1967), and naphthol blue-black specifically stains soluble or reserve proteins dark blue (Fisher, 1968). With this staining technique, polyphenols are a brown color. Cover slips were mounted with Entellan mounting medium (Ref: 1.07961.0500, Merck) and dried at room temperature.
Cacao zygotic embryos were also fixed in glutaraldehyde 3 % for 24 hours in phosphate buffer (pH 7.2 at 0.2 M) and free hands cuts were made. Fresh slides were mounted with glicerine. Fresh and permanent slides were examined on a Nikon eclipse 80i light microscope (Nikon Corporation, Kanagawa, Japan) and digital photographs were taken using a Leica DC 200 digital camera (Leica Microsystems AG, Wetzlar, Germany).
Transmission Electron Microscopy (TEM)
To verify the presence and distribution of polyphenols in tissues, we used transmission electron microscopy. Samples were fixed in glutaraldehyde 3 % in buffer phosphate 2 M pH 7.2 and refrigerated (4 ºC) for 24 hours. Samples were washed in glutaraldehyde % for 24 hours in buffer phosphate 2 M pH 7.2 for three times. Samples were then fixed in osmium tetroxide (1 %) prepared fresh over1 hour at room temperature and washed in distilled water three times. Afterwards, samples were dehydrated in ethanol 25 % and 50 % at each step and in ethanol 70 % with uranile acetate overnight. Samples were then dehydrated in ethanol 90 % and 100 % for 15 minutes at each concentration. Imbibition in pure acetone was conducted three times at 15-minutes intervals. Imbibition in pure acetone: Spurr resin (1:1) was conducted for a half hour. Later, the samples were imbibed in pure Spurr resin for 1 hour and added to the molds. Polymerization was achieved at 62 ºC for 24 hours. Later, samples were cut in a PawerTome XL ultramicrotome in slides of 70 µm. Samples were processed in a JEOL JEM 1011 Transmission electron microscope adapted with a Gatan camera of 11 mpx.
RESULTS
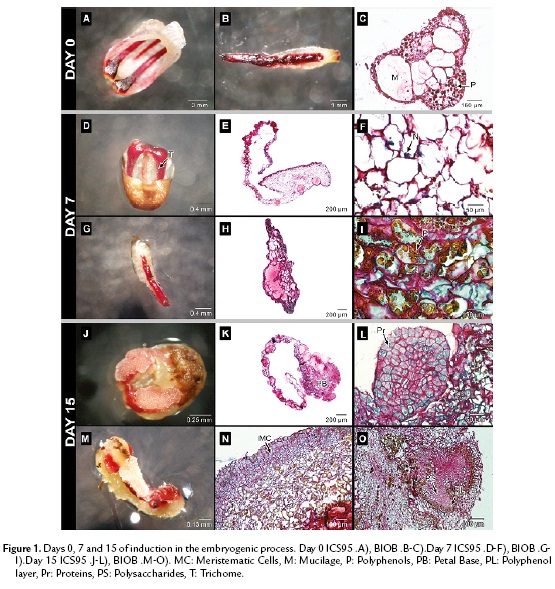
Figure 1A and 1B show the structure of staminodes and petals before the culture. Both, the petals in ICS95 and the staminodes in BIOB comprised distinct layers of epidermal cells, parenchyma cells, vascular bundles and large vesicles full of mucilage. In this stage, the occurrence of polyphenolic cells (brown) was common in both explants around vascular tissue and distributed randomly (Fig. 1C).
After seven days of culture, petal and staminode surfaces appeared smooth with large numbers of long trichomes in response to the medium (Fig. 1D, 1G). The cells were characterized by a large quantity of polysaccharides and sub-epidermal and perivascular cells showed strong staining of the nucleus, leading to significant mitotic activity, mainly in petals. The cells were rich in mucilage and polysaccharides, and the latest were observed as granular shaped structures (Fig. 1E, 1F, 1H). Polyphenols were distributed randomly in both explants (Fig. 1I).
Fifteen days after the establishment, callus formation was observed in both explants toward the periphery (Fig.1J, 1M). In ICS95, this event was localized firstly in the petal base (Fig. 1K). In BIOB, the calli formation is clear all over the periphery of the staminode (Fig. 1N).
In both explants, the tissue was divided into zones differentiated by cell type. The meristematic region had a defined separation formed by thin cell walls, with dense cytoplasm which were separated from the surrounding larger and more vacuolated cells in the tissue (Fig. 1K, 1N). From the explant base toward the periphery, swollen cells were observed emerging from the epidermal cells and the progressive synthesis of storage molecules occurred. The cytoplasm of meristematic cells contained starch and proteins as shown by specific coloration for both explants. During this period, a globular structure from petals, with a high meristematic activity was observed, originating from epidermal cells for ICS95 (Fig. 1L). In BIOB, nodular calli with meristematic cells were formed from the epidermal cells, showing anticlinal division with high protein content (Fig. 1N). Additionally, inside the tissue, round and dense structures rich in polysaccharides were present, which were surrounded by a thin layer of cells containing polyphenols (Fig.e 1O).
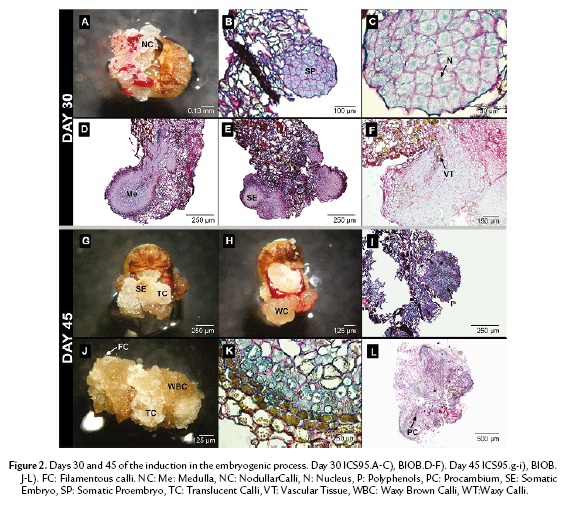
On the thirtieth day of culture, nodules of granular calli containing meristematic cells were visible in the periphery in ICS95 (Fig. 2A). These periclinal cells accumulate a large protein quantity reserve in the central nuclei, which occupies the main part of the cellular volume. Later, meristematic cells developed embryonic features (Fig. 2B). A globular structure was observed with high protein content, but it was not possible to differentiate either a medulla or an epidermal layer (Fig. 2C).
In BIOB, the embryo formation was raised from an epidermal cells as well as from parenchymal cells inside the callus. The embryo showed different layers varying in cell content. It was possible to differentiate a medulla with larger cells in the inner part from the periphery, showing a suspensor-like growth at the micropilar pole (Fig. 2D). Occasionally, centers of meristematic activity were found in the highly vacuolated inner region. The cell indicated high polysaccharide and protein content (Fig. 2E). At this stage, the embryo cells started to elongate and a diffuse vascular tissue was present (Fig. 2F). Polyphenols were only distributed in the periphery of the embryo structure.
On the forty-fifth day, two morphological types of calli were clearly recognizable in ICS95. The first type consisted of round, translucent glassy-white cells (TC); mainly parenchymal cells were observed under the scope. Few somatic embryos were developed of this type of callus (Fig. 2G). The second type consisted of waxy-yellow cells (WC), which were not found in association with somatic embryos (Fig. 2H). Globular embryos that formed in ICS95 did not differentiate any further. Instead, polyphenols, polysaccharides and proteins were distributed randomly inside these cells (Fig. 2I).
For BIOB, three types of calli were recognized: a vitreous granular-white calli (TC); waxy granular brown calli (WBC); and filamentous white calli (FC) (Fig. 2J). Embryos always originated from brown granular calli (WBC). Most of the polyphenols in the embryo were only restricted to the periphery (Fig. 2K). The embryos in this step were elongated and exhibited a definite vascular tissue associated with polyphenolic cells and proteins were more concentrated in the apical zone of the embryo (Fig. 2L).
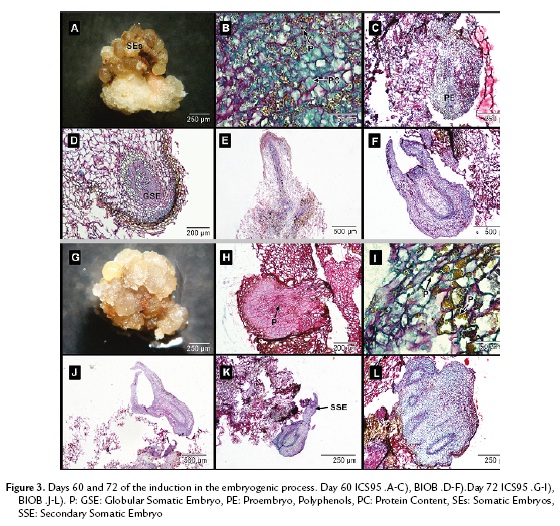
In ICS95, sixty days after establishment, the somatic embryos took on a dark brown color (Fig. 3A) and were full of polyphenols mixed with proteins and polysaccharides (Fig. 3B). The tissue did not present any further divisions, which indicated the somatic embryogenic process was stopped (Fig. 3C).
In BIOB embryos, a remarkable change was observed compared to ICS95 embryos. Polysaccharide content diminished, while proteins became the main content in the embryos. It was possible to see a medulla and epidermal layer inside them. The somatic embryos presented a definite polarity, leaf primordia and apical meristem. The embryonic masses were spatially isolated from the surrounding cells, which confirmed that somatic embryogenesis occurs in each mass independently. Subsequently, these cellular groups developed into mature somatic embryos.
In BIOB, polyphenols were not present inside the tissue but distributed in the periphery of the epidermal embryo cells. At this stage, the primary somatic embryos acquired a protoderm and procambial bundles and then shoot and root meristems, going through the globular (Fig. 3D), torped-shaped (Fig. 3E) and cotyledonary stages (Fig. 3F) that resemble zygotic embryo development. During this process, cortical parenchyma of young embryos were still in the process of differentiation. The somatic embryos contained mainly protein reserves in their cortical and medulla parenchyma.
After seventy-two days, the waxy-yellow embryos turned a waxy brown color in ICS95 (Fig. 3G). It was not possible to see an epidermal layer or cortical cells in any of the globular embryos, and they were full of polysaccharides (Fig. 3H). The polyphenols and proteins were also present and distributed randomly (Fig. 3I). Finally, the embryos lost their regeneration capability.
In BIOB, somatic embryos were isolated from maternal tissue, confirming the parameter of independence (Fig. 3J). Some of the formed somatic embryos became sites of active adventitious embryogenesis (Fig. 3K). The process of secondary embryo production took place at the periphery of preexisting embryos with to a budding process with no prior callus. These embryos presented a high protein content similar to that of fused embryos (Fig. 3L).
To confirm the presence and distribution of polyphenols in somatic embryos, we used TEM. This technique allowed us to observe peripheral localization (epidermal cells) of the polyphenols in cacao zygotic embryos (Fig. 4A), coinciding with the somatic embryos of the regenerating variety BIOB (Fig. 4B). For the non-regenerating variety ICS95, the polyphenol distribution were randomly (Fig. 4C). To conclude all results are summarized in the table 1.
DISCUSSION
One major problem of plant regeneration from somatic embryos is their limited amount of available storage compounds, lacking the extra-embyonal reservoir zygotic embryos have at their disposal (Alferman et al., 2003). Most of papers focused in demonstrating those compounds limitations, however, their distribution is not taking into account.
Somatic embryo production in cacao is a complex and difficult process mainly because of the wide range of genotypes which show diverse responses to the tissue culture treatments (Maximova et al., 2008). The responses may vary during the embryogenic processes and thus may be positive during the early stages such as the callus formation and callus differentiation stages and negative during the later stages of maturation (Janick et al., 1980; Pence, 1995).
Regarding the induction of cacao somatic embryogenesis, several authors have studied the response of floral explants to callus formation, obtaining different results according to the methodology used (Sondahl et al., 1993; Li et al., 1998; López-Baez et al., 2001). Similarly, in this work, although all treatments induced callus formation, in both staminodes and petals, the response varied depending on the genotype and culture media used (Fig. 1J, 1M). Similar responses have been reported in Coffea spp, Cydonia oblonga Mill and Robinia pseudoacacia L, among other crops (Sreenath et al., 1995; Han and Park, 1999; Etienne et al., 1999; Fisichella et al., 2000).
In this work, at the end of the callogenesis step, the calli were made up of granular nodules comprising meristematic cell groups of various sizes surrounded by parenchymal cells. In the expression medium, a specific area was observed forming well-developed meristematic zones. One month later, some meristematic cells acquired embryonic characteristics similar to those found in the cells of young zygotic embryos, a result that agreed with Alemanno et al. (1996) (Fig. 2B, 2E). The cells had a dense cytoplasm, a large strongly colored nucleus rich in proteins and some starch bodies similar to Kononowicz et al. (1984) working with cacao. The meristematic activity was observed early in day seven for ICS95 (Fig. 1F). Some of the clustered cells became clearly differentiated from neighboring cells, and finally the embryos appeared (Fig. 2B). These embryos did not go into further differentiation, and they were rich in polysaccharides and polyphenols, which were randomly distributed. In the BIOB variety, the embryos had high protein content and acquired a protodermis (characterized for the presence of polyphenols), procambial bundles and apical shoot and root meristems, passing through all stages and thus mimicking ontogenesis in zygotic embryos (Fig. 2L).
We hypothesize possible answers to plant regeneration can reside in the storage compounds, not only in their presence but also in their distribution. So, in relation to the polysaccharide and protein content in somatic embryos Noah et al. (2013), evaluated the cacao SE proteome and made a functional classification of the identified proteins. They highlighted seven categories: (1) carbohydrate metabolism; (2) energy metabolism; (3) amino acid metabolism; (4) genetic information processing; (5) cellular processes; (6) stress response; and (7) defense. As described above, embryogenesis is a complex developmental process which is extensively based on carbohydrate metabolism (including sugar conversion), as previously reported in Picea glauca (Iraqi and Tremblay, 2001), Cyclamen persicum (Rode et al., 2012) and in Phoenix dactylifera (Sghaier-Hammami et al., 2009). The explanation for extensive carbohydrate metabolism is the heavy energy demand required for metabolic processes that occur during cell division and elongation (Kroon and Williams, 1999). At the 15th day, ICS95 embryos were formed, where protein and polysaccharide presence were kept constant during embryo induction. In BIOB, during the last stages of the maturation process, somatic embryos were visible from day 30, accompanied by a changes in cell content such as a reduction of polysaccharides and the high protein content occupying the main cell volume.
Interestingly, Noah et al. (2013) demonstrated that SE of Theobroma cacao may undergo precocious mobilization of storage compounds which may be the reason for the lesser vigor of derived plantlets compared to those originating from zygotic embryogenesis. This result is congruent with ours, where meristematic activity and the mobilization of storage reserve occurred earlier in ICS95 than BIOB. Consequently, the induced somatic embryos in ICS95 did not undergo differentiation.
It has been reported that cacao SE proteomes include a higher abundance of proteins involved in other metabolic pathways of carbohydrate metabolism, such as lactoylglutathionelyase, malate synthase, a putative L-galactose-1-phosphate phosphatase and malate dehydrogenase (Noah et al., 2013). Furthermore, oxidative phosphorylation, represented by high contents of NADH-ubiquinone oxidoreductase, ATP synthase and flavoprotein, indicate that these embryos undergo extensive oxidation processes (Griffiths, 1958; Omokolo et al., 1997; Sanbongi et al., 1998).
Additional factors associated with recalcitrance in the process of in vitro development have been described, including "whole plant physiology of the donor" in which life cycle, reproduction, explant choice and rejuvenation capability all affect the tissue cultures. Other factors is "in vitro plant stress physiology" have also been considered, including production of ethylene by plant tissue cultures, sensibility of tissues to plant growth regulators and the oxidation of explants (Li et al., 1998; Benson, 2000).
Under stressed conditions, plant cells change their metabolism, growth and development in order to be suited for new environment conditions (Dahleen and Bregitzer, 2002). Somatic cells of plants require several stress factors to switch their developmental program to a specific physiological state that allows the reprogramming of gene expression towards acquisition of embryonic competence (Pasternak et al., 2002). In Theobroma cacao, a set of stress factors encompassing wounding, salt content and several growth regulators such as TDZ, Kinetin, ANA are required to turn somatic cells of floral explant into competent embryonic callus cultures (Li et al., 1998; Traore et al., 2003). Other plant growth regulators, such as 2,4-D, (used for both varieties), are especially recognized as oxidative stress inducers, and auxin-induced proteins are also involved in somatic embryogenesis (Omokolo et al., 1997).
An explanation for the lack of regeneration of the ICS95 embryos could be related to the presence of Thidiazuron (TDZ) in the medium. According to Quainoo and Dwomo (2012b), cacao tissue is highly sensitive to TDZ concentration because ethylene levels in cells can increase and affect the metabolism of endogenous cytokinins. Moreover, in this variety, organized apical meristems were absent, like in cacao somatic embryogenesis as reported by Dodeman et al., (1997) as well as with soybean by Barwale et al., (1986) and Vitis longii by Gray and Mortensen (1987). Goebel-Tourand et al., (1993) suggested that somatic embryogenesis implies several interactions where the alteration in one factor may triggers continuos uncommon abnormal events. However, with the BIOB variety, the organization of apical meristematic zones were identified in concordance with Dodeman (1997), where once the primary meristems are formed, embryogenesis sensu stricto can be considered as completed and the subsequent stages of development are focused on the phenomena associated with maturation.
In this study, vitreous granular-white calli and brown granular calli were associated with embryo formation in the expression medium (30 days after establishment). However, embryos derived from white calli did not undergo differentiation. According to Juarez (2012), the brown calli are the type where somatic embryogenesis successfully occurs in cacao. This assumption is in accordance with a result obtained in cotton, where the fawn-brown calli showed better potential to develop into somatic embryos (Wang et al., 2006).
The oxidation of explants in culture initiation is a frequent cause of early in vitro recalcitrance in tissues because of a high phenolic content (Anthony et al., 1999). The problem is most prevalent in woody plant tissues (like cacao), as they have high levels of phenols associated with secondary thickening and lignification and, in other cases, with the production of secondary metabolites. The oxidation of phenolic compounds could be associated with factors such as wound response, sterilization process and specific components of the tissue culture media (e.g., metal cations), among others (Pearce et al., 1998; Benson, 2000; Lattanzio et al., 2006).
In this study, polyphenols were present in the tissues of both regenerating and non-regenerating embryos. However, the distribution of polyphenols in these tissues was differently established. The peripheral distribution found in somatic embryos of the BIOB variety agreed with the pattern found in zygotic embryos (Fig. 2K, 4A). Moreover, consistent with Alemanno et al. (2003), somatic embryos that regenerated were free of inner polyphenols, and only small amounts were distributed in the periphery of the embryo epidermal layer, while in the non-regenerating variety, embryogenic calli were compact and such structures contained polyphenols randomly distributed across all tissues. Polyphenols were abundant in staminodes and petals on day 0, but their distribution changed during the development process according to genotype and embryogenic capacity. Positioning of polyphenols externally was observed in zygotic embryos of cacao (Fig. 4A) and has been observed in other plants (Zobel et al., 1989; Salatino et al., 1993).
The polyphenol distribution pattern found in somatic embryos of the regenerating variety could indicate that, under the in vitro conditions evaluated, the genetic reprogramming of tissues was coincident with that found in cacao zygotic embryogenesis, which explains the regenerative capability of this variety. Therefore, it was speculated that the distribution of phenolic compounds to periphery in somatic embryos might reflect the natural process in zygotic embryos, which polyphenols go through the cell wall, and store in the intercellular spaces outside the cell, to inhibit the pathogens invading from the apoplast pathway and start the first defense barrier in time after the plant was harmed or infected. As to the abundant phenolic compounds in the vascular parenchyma cells, it might perform important protective effect on preventing the pathogen from spreading via vessels and guaranteeing the normal transportation of the nutrients in the phloem (Li et al., 2012). The changes in protein and polysaccharides are additional evidence in support of this hypothesis.
CONCLUSIONS
Embryogenic capacity, therefore, seem to be associated with a balanced concentration and distribution of polyphenols. High concentrations are associated with the non-regenerating response of somatic embryos (Alemanno, 2003) and histolocalization of phenolic compounds could be a marker to distinguish regenerating and non-regenerating embryos. Further early screening of phenol content in the tissue culture will help in predicting developmental events in cacao. Similar studies are required in other cacao genotypes and related species in order to confirm if the distribution of polyphenols can serve as a marker of regeneration in somatic embryos.
ACKNOWLEDGMENTS
We thank the Colombian Ministry of Agriculture (Code MADR 2008E3776) and Compañía Nacional de Chocolates and Universidad de Antioquia for the research support.
CONFLICT OF INTERESTS
There is no conflict of interests between Ministry of Agriculture (Code MADR 2008E3776) and Compañía Nacional de Chocolates and Universidad de Antioquia for the research.
REFERENCES
Alemanno L, Berthouly MA, Michaux-Ferriere N. A comparison between Theobroma cacao L. zygotic embryogenesis and somatic embryogenesis from floral explants. In Vitro Cell Dev Bio. 1997;33:163-172.
Alemanno L, Berthouly M, Michaux-Ferriere N. Histology of somatic embryogenesis from floral tissues cacao. Plant Cell Tis Org Cult. 1996;46:187-194.
Alemanno L, Ramos T, Gargadenec A, Andary C, Ferriere N. Localization and identification of phenolic compounds in Theobroma cacao L. somatic embryogenesis. Ann Bot. 2003;92:613-23.
Alferman AW, Petersen M, Fuss E. Production of natural products by plant cell biotechnology: Results problems and perspectives. In: Laimer M, Rücker W, editors. Plant Tissue Culture-100 years since Gottlieb Haberlandt. Wien: Springer. 2003; p. 153-166.
Anthony JM, Senaratna T, Dixon KW, Sivasithamparam K, Bunn E. In vitro regeneneration of recalcitrant Australian plants. In Vitro Cell Dev Biol. 1999;35:53-A;P-1062.
Bailey-Serres J, Mittler R. The Roles of Reactive Oxygen Species in Plant Cells. Plant Physiol. 2006;141(2):311. Doi:10.1104/pp.104.900191.
Barwale UB, Kerns HR, Wildhom JM. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta. 1986;164:473-81.
Benson E. Special symposium: In vitro plant recalcitrance. In vitro plant recalcitrance: An introduction. In Vitro Cell Dev Biol Plant. 2000;36:141-148.
Dahleen LS, Bregitzer P. An improved media system for high regeneration rates from barley immature embryo- derived callus cultures of commercial cultivars. Crop Sci. 2002;42:934-938.
Dangou JS, Hocher V, Ferrière N, Fulcheri C, Morard P, Alemanno L. Histological and biochemical characterization of Theobroma cacao L. endosperm during seed development. Seed Sci Res. 2007;12:91-100.
Driver D, Kuniyuki, D. In vitro propagation of Paradox walnut rootstock. Hort Science. 1984;19:507-509.
Dodeman VL, Ducreux G, Kreis M. Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot. 1997;48:1493-1509.
Etienne H, Barry-Etienne D, Vásquez N, Berthouly M. Aportes de la Biotecnología al Mejoramiento Genético del Café: El Ejemplo de la Multiplicación por Embriogénesis Somática de Híbridos F1 en América Central. In: Bertrand B, Rapidel B, editors. Costa Rica, San José: Desafíos de la Caficultura en Centroamérica; 1999. p. 457-499.
Fang J, Wetten A, Hadley P. Cryopreservation of cacao Theobroma cacao L. somatic embryos for long-term germplasm storage. Plant Sc. 2004;3:669-675.
Figueira A, Janick J. Somatic embryogenesis in cacao. Theobroma cacao L. In: Jain S, Gupta P, Newton R, editors. Somatic embryogenesis in woody plants, vol. 2-Angiosperms. Netherlands: Kluwer Academic Publishers; 1995. p. 291-310.
Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemie. 1968;16:92-96.
Fisichella M, Silvi E, Morini S. Regeneration of Somatic Embryos and Roots from Quince Leaves Cultured on Media with Diferent Macroelement Composition. Plant Cell Tis Org Cul. 2000;63:101-107.
Fontanel A, Gire-Bobin S, Labbé G, Favereau P, Álvarez M, Rutte S, et al. In vitro multiplication and plant regeneration of Theobroma cacao L. via stable embryogenic calli. 10 IAPTC Congress, Plant Biotechnology, 2002.
Goebel-Tourand I, Mauro M, Sossountazov C, Miginiac M, Deloire E. Arrest of somatic embryo development in grape-vine: Histological characterization and the effect of ABA, BAP and zeatin in stimulating plantlet development. Plant Cell Tiss Org Cult. 1993;33:91-103.
Gray DJ, Mortensen JA. Initiation and maintenance of long-term somatic embryogenesis from anthers and ovaries of Vitislongii 'Microsperma'. Plant Cell Tiss Org Cult, 1987;9:73-80.
Griffiths LA. Phenolic Acids and Flavonoids of Theobroma cacao L. Separation and Identification by Paper Chromatography. Biochem J. 1958;1:120-125.
Guiltinan M, Young A, Miller C, Antunez de Mayolo G, Swanson JD, Pishak S, Maximova S. Cacao Tissue Culture. USA: Protocol Book; 2003. p. 32.
Han K, Park Y. Somatic embryogenesis in black locust. Robinia pseudoacacia L. somatic embryogenesis in woody plants. Forest Sci. 1999;5:149-161.
Iraqi D, Tremblay FM. Analysis of carbohydrate metabolism enzymes and cellular contents of sugar and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J Exp Bot. 2001;52:2301-2311.
Janick J, Pence V, Hasegawa P. Initiation and Development of Asexual Embryos of Theobroma cacao L. in vitro. Z Pflanzenphysiol. 1980;98(1):1-14. Doi: 10.1016/S0044-328X(80)80214-5.
Juarez D. Somatic embryogenesis and long term conservation of cacao. Theobroma cacao L. germ plasm. University of Helsinki, 2012. p.250.
Kononowicz H, Kononowicz AK, Janick J. Asexual Embryogenesis via Callus of Theobroma cacao. Z Pflanzenphysiol. 1984;113(4):347-358. Doi:1 0.1016/S0044-328X(84)80041-0.
Kroon PA, Williams G. Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agr. 1999;79:355-361.
Lattanzio V, Lattanzio VMT, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem Adv Res. 2006;23-67.
Li Z, Tang T, Liang S, Ning X, Bai M, Wu H. The Synthesis and Storage Sites of Phenolic Compounds in the Root and Rhizome of Echinacea purpurea. Am J Plant Sci. 2012;3:551-558.
Li Z, Traore A, Maximova S, Guiltinan MJ. Somatic embryogenesis and plant regeneration from floral explants of cacao. Theobroma Cacao L. using thidiazuron. In Vitro Cell Dev Biol, 1998;34:293-299.
Loyola V, Vasquez F. Plant Cell Culture Protocols. Second Edition. USA: Humana Press Inc; 2006. p. 425.
López-Baez O, Moreno-Marginez L, Pacheco-Rodas S. Avances en Propagación de Cacao- Theobroma cacao- por Embriogénesis Somática en México. International Workshop on new Technologies and Cacao Breeding. Malaysa; 2001. p. 163-176.
Martoja R, Martoja PM. Initiation aux Techniques de l'Histologie animale. 1967. Paris: Masson p. 345.
Maximova SN, Alemanno L, Young A, Ferriere N, Traore A, Guiltinan MJ. Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis. In Vitro Cell Dev Biol Plant. 2002;38:252-259.
Maximova SN, Young A, Pishak S, Guiltinan MJ. Field performance of Theobroma cacao L. plants propagated via somatic embryogenesis. In Vitro Cell Dev Biol Plant. 2008;44:487-493.
Merkle S, Parrott W, Flinn B. Morphogenic aspects of somatic embryogenesis. In: Thorpe TA, editor. In Vitro Embryogenesis in Plants. Dordrecht: Kluwer Academic Publishers; 1995. p. 155-204.
Noah AM, Niemenak N, Sunderhaus S, Haase C, Omokolo DN, Winkelmann T, Braun H-P. Comparative proteomic analysis of early somatic and zygotic embryogenesis in Theobroma cacao L. J Proteomics. 2013;78:123-33.
Omokolo D, Tsala G, Niemenak N. Phenol Content, Acidic Peroxidase and IAA-oxidase during somatic embryo in Theobroma cacao L. Bio Plant. 1997;39:337-347.
Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, et al. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of Alfalfa. Plant Physiol. 2002;129:1807-19.
Pearce G, Marchand PA, Griswold J, Lewis NG, Ryan C. Accumulation of feruloyltyramine and p-coumaroyltyramine in tomato leaves in response to wounding. Phytochemestry. 1998;47:659-664.
Pence V. Somatic Embryogenesis in Cacao. Theobroma cacao. Biotechnol Agric For. 1995;30:455-467.
Quainoo KA, Dwomon I. The Effect of Abscisic Acid in the Conversion of Cacao Somatic Embryos into Plantlets. Front Plant Sci. 2012a;2(2):6-10. Doi:10.5923/j.fs.20120202.02.
Quainoo KA, Dwomon I. The effect of TDZ and 2,4D concentrations on the induction of somatic embryo and embryogenesis in different cacao genotypes. J Plant Stud. 2012b;1(1):72-78. Doi: 10.5539/jps.v1n1p72.
Rode C, Lindhorst K, Braun HP, Winkelmann T. From callus to embryo — a proteomic view on the development and maturation of somatic embryos in Cyclamen persicum. Planta. 2012;35:995-1101. Doi:10.1007/s00425-011-1554-1.
Salatino A, Kraus J E, Salatino MLF. Contents of tannins and their hystological localization in young and adult part of Struthanthus vulgaris Mart. Loranthaceae. Ann Bot. 1993;70:409-457. Doi:10.1006/anbo.1993.1126.
Sanbongi C, Osakabe N, Natsume M, Takizawa T, Gomi S. Antioxidative Polyphenols Isolated from Theobroma cacao. J Agric Food Chem. 1998;8561:454-457.
Sghaier-Hammami B, Drira N, Jorrín-Novo VJ. Comparative 2-DE proteomic analysis of date palm (Phoenix dactylifera L.) somatic and zygotic embryos. J Prot. 2009;1:161-77.
Sondahl M, Liu S, Bellato C, Bragin A. Cacao somatic embryogenesis. Acta Hort. 1993;336:245-248.
Sreenath H, Shanta H, Harinat K, Naidu. Somatic Embryogenesis from Integument .perisperm. Cultures of Coffee. Plant Cell Rep. 1995;14: 670-673.
Suarez M, Bozhkov P. Plant Embryogenesis. USA: Humana Press Inc; 2008. p. 179.
Traore A, Maximova SN, Guiltinan MJ.Micropropagation of Theobroma cacao L. using somatic embryo-derived plants. In Vitro Cell Dev Biol Plant. 2003;39:332-337.
Urrea A, Gallego A, Atehortúa L. Regeneración vía embriogénesis somática de una variedad colombiana élite de Theobroma cacao L. Rev Col Biot. 2011;13:39-50.
Wang YX, Wang XF, Ma ZY, Zhang GY, Han GY. Somatic embryogenesis and plant regeneration from two recalcitrant genotypes of Gossypium hirsutum L. Agri Sci China. 2006;5(5):323-329.
Zobel A, Kura M, Tykarska T. Cytoplasmic and apoplastic location of phenolic compounds in the covering tissue of the Brassica napus radicle between embryogenesis and germination. Ann Bot. 1989;64:149-157.
Referencias
Alemanno L, Berthouly MA, Michaux-Ferriere N. A comparison between Theobroma cacao L. zygotic embryogenesis and somatic embryogenesis from floral explants. In Vitro Cell Dev Bio. 1997;33:163–172.
Alemanno L, Berthouly M, Michaux-Ferriere N. Histology of somatic embryogenesis from floral tissues cacao. Plant Cell Tis Org Cult. 1996;46:187–194.
Alemanno L, Ramos T, Gargadenec A, Andary C, Ferriere N. Localization and identification of phenolic compounds in Theobroma cacao L. somatic embryogenesis. Ann Bot. 2003;92:613–23.
Alferman AW, Petersen M, Fuss E. Production of natural products by plant cell biotechnology: Results problems and perspectives. In: Laimer M, Rücker W, editors. Plant Tissue Culture-100 years since Gottlieb Haberlandt. Wien: Springer. 2003; p. 153-166.
Anthony JM, Senaratna T, Dixon KW, Sivasithamparam K, Bunn E. In vitro regeneneration of recalcitrant Australian plants. In Vitro Cell Dev Biol. 1999;35:53-A;P-1062.
Bailey-Serres J, Mittler R. The Roles of Reactive Oxygen Species in Plant Cells. Plant Physiol. 2006;141(2):311. Doi:10.1104/pp.104.900191.
Barwale UB, Kerns HR, Wildhom JM. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta. 1986;164:473-81.
Benson E. Special symposium: In vitro plant recalcitrance. In vitro plant recalcitrance: An introduction. In Vitro Cell Dev Biol Plant. 2000;36:141–148.
Dahleen LS, Bregitzer P. An improved media system for high regeneration rates from barley immature embryo- derived callus cultures of commercial cultivars. Crop Sci. 2002;42:934–938.
Dangou JS, Hocher V, Ferrière N, Fulcheri C, Morard P, Alemanno L. Histological and biochemical characterization of Theobroma cacao L. endosperm during seed development. Seed Sci Res. 2007;12:91-100.
Driver D, Kuniyuki, D. In vitro propagation of Paradox walnut rootstock. Hort Science. 1984;19:507-509.
Dodeman VL, Ducreux G, Kreis M. Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot. 1997;48:1493– 1509.
Etienne H, Barry-Etienne D, Vásquez N, Berthouly M. Aportes de la Biotecnología al Mejoramiento Genético del Café: El Ejemplo de la Multiplicación por Embriogénesis Somática de Híbridos F1 en América Central. In: Bertrand B, Rapidel B, editors. Costa Rica, San José: Desafíos de la Caficultura en Centroamérica; 1999. p. 457-499.
Fang J, Wetten A, Hadley P. Cryopreservation of cacao Theobroma cacao L. somatic embryos for long-term germplasm storage. Plant Sc. 2004;3:669-675.
Figueira A, Janick J. Somatic embryogenesis in cacao. Theobroma cacao L. In: Jain S, Gupta P, Newton R, editors. Somatic embryogenesis in woody plants, vol. 2-Angiosperms. Netherlands: Kluwer Academic Publishers; 1995. p. 291–310.
Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemie. 1968;16:92–96.
Fisichella M, Silvi E, Morini S. Regeneration of Somatic Embryos and Roots from Quince Leaves Cultured on Media with Diferent Macroelement Composition. Plant Cell Tis Org Cul. 2000;63:101–107.
Fontanel A, Gire-Bobin S, Labbé G, Favereau P, Álvarez M, Rutte S, et al. In vitro multiplication and plant regeneration of Theobroma cacao L. via stable embryogenic calli. 10 IAPTC Congress, Plant Biotechnology, 2002.
Goebel-Tourand I, Mauro M, Sossountazov C, Miginiac M, Deloire E. Arrest of somatic embryo development in grape-vine: Histological characterization and the effect of ABA, BAP and zeatin in stimulating plantlet development. Plant Cell Tiss Org Cult. 1993;33:91-103.
Gray DJ, Mortensen JA. Initiation and maintenance of long- term somatic embryogenesis from anthers and ovaries of Vitislongii ‘Microsperma’. Plant Cell Tiss Org Cult, 1987;9:73-80.
Griffiths LA. Phenolic Acids and Flavonoids of Theobroma cacao L. Separation and Identification by Paper Chromatography. Biochem J. 1958;1:120–125.
Guiltinan M, Young A, Miller C, Antunez de Mayolo G, Swanson JD, Pishak S, Maximova S. Cacao Tissue Culture. USA: Protocol Book; 2003. p. 32
Han K, Park Y. Somatic embryogenesis in black locust. Robinia pseudoacacia L. somatic embryogenesis in woody plants. Forest Sci. 1999;5:149-161.
Iraqi D, Tremblay FM. Analysis of carbohydrate metabolism enzymes and cellular contents of sugar and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J Exp Bot. 2001;52:2301–2311.
Janick J, Pence V, Hasegawa P. Initiation and Development of Asexual Embryos of Theobroma cacao L. in vitro. Z Pflanzenphysiol. 1980;98(1):1–14. Doi: 10.1016/S0044- 328X(80)80214-5
Juarez D. Somatic embryogenesis and long term conservation of cacao. Theobroma cacao L. germ plasm. University of Helsinki, 2012. p.250.
Kononowicz H, Kononowicz AK, Janick J. Asexual Embryogenesis via Callus of Theobroma cacao. Z Pflanzenphysiol. 1984;113(4):347–358. Doi:10.1016/ S0044-328X(84)80041-0
Kroon PA, Williams G. Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agr. 1999;79:355–361.
Lattanzio V, Lattanzio VMT, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem Adv Res. 2006;23-67.
Li Z, Tang T, Liang S, Ning X, Bai M, Wu H. The Synthesis and Storage Sites of Phenolic Compounds in the Root and Rhizome of Echinacea purpurea. Am J Plant Sci. 2012;3:551-558.
Li Z, Traore A, Maximova S, Guiltinan MJ. Somatic embryogenesis and plant regeneration from floral explants of cacao. Theobroma Cacao L. using thidiazuron. In Vitro Cell Dev Biol, 1998;34:293–299.
Loyola V, Vasquez F. Plant Cell Culture Protocols. Second Edition. USA: Humana Press Inc; 2006. p. 425.
López-Baez O, Moreno-Marginez L, Pacheco-Rodas S. Avances en Propagación de Cacao- Theobroma cacao- por Embriogénesis Somática en México. International Workshop on new Technologies and Cacao Breeding. Malaysa; 2001. p. 163–176.
Martoja R, Martoja PM. Initiation aux Techniques de l’Histologie animale. 1967. Paris: Masson p. 345.
Maximova SN, Alemanno L, Young A, Ferriere N, Traore A, Guiltinan MJ. Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis.
In Vitro Cell Dev Biol Plant. 2002;38:252–259. Maximova SN, Young A, Pishak S, Guiltinan MJ. Field performance of Theobroma cacao L. plants propagated via somatic embryogenesis. In Vitro Cell Dev Biol Plant. 2008;44:487–493.
Merkle S, Parrott W, Flinn B. Morphogenic aspects of
somatic embryogenesis. In: Thorpe TA, editor. In Vitro Embryogenesis in Plants. Dordrecht: Kluwer Academic Publishers; 1995. p. 155-204.
Noah AM, Niemenak N, Sunderhaus S, Haase C, Omokolo DN, Winkelmann T, Braun H-P. Comparative proteomic analysis of early somatic and zygotic embryogenesis in Theobroma cacao L. J Proteomics. 2013;78:123–33.
Omokolo D, Tsala G, Niemenak N. Phenol Content, Acidic Peroxidase and IAA-oxidase during somatic embryo in Theobroma cacao L. Bio Plant. 1997;39:337–347.
Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, et al. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast- derived cells of Alfalfa. Plant Physiol. 2002;129:1807–19.
Pearce G, Marchand PA, Griswold J, Lewis NG, Ryan C. Accumulation of feruloyltyramine and p-coumaroyltyramine in tomato leaves in response to wounding. Phytochemestry. 1998;47:659–664.
Pence V. Somatic Embryogenesis in Cacao. Theobroma cacao. Biotechnol Agric For. 1995;30:455-467.
Quainoo KA, Dwomon I. The Effect of Abscisic Acid in the Conversion of Cacao Somatic Embryos into Plantlets. Front Plant Sci. 2012a;2(2):6–10. Doi:10.5923/j.fs.20120202.02
Quainoo KA, Dwomon I. The effect of TDZ and 2,4D concentrations on the induction of somatic embryo and embryogenesis in different cacao genotypes. J Plant Stud. 2012b;1(1):72-78. Doi: 10.5539/jps.v1n1p72
Rode C, Lindhorst K, Braun HP, Winkelmann T. From callus to embryo — a proteomic view on the development and maturation of somatic embryos in Cyclamen persicum. Planta. 2012;35:995–1101. Doi:10.1007/s00425-011- 1554-1.
Salatino A, Kraus J E, Salatino MLF. Contents of tannins and their hystological localization in young and adult part of Struthanthus vulgaris Mart. Loranthaceae. Ann Bot. 1993;70:409-457. Doi:10.1006/anbo.1993.1126
Sanbongi C, Osakabe N, Natsume M, Takizawa T, Gomi S. Antioxidative Polyphenols Isolated from Theobroma cacao. J Agric Food Chem. 1998;8561:454–457.
Sghaier-Hammami B, Drira N, Jorrín-Novo VJ. Comparative 2-DE proteomic analysis of date palm (Phoenix dactylifera L.) somatic and zygotic embryos. J Prot. 2009;1:161–77.
Sondahl M, Liu S, Bellato C, Bragin A. Cacao somatic embryogenesis. Acta Hort. 1993;336:245–248.
Sreenath H, Shanta H, Harinat K, Naidu. Somatic Embryogenesis from Integument .perisperm. Cultures of Coffee. Plant Cell Rep. 1995;14: 670-673.
Suarez M, Bozhkov P. Plant Embryogenesis. USA: Humana Press Inc; 2008. p. 179.
Traore A, Maximova SN, Guiltinan MJ.Micropropagation of Theobroma cacao L. using somatic embryo-derived plants. In Vitro Cell Dev Biol Plant. 2003;39:332–337.
Urrea A, Gallego A, Atehortúa L. Regeneración vía embriogénesis somática de una variedad colombiana élite de Theobroma cacao L. Rev Col Biot. 2011;13:39–50.
Wang YX, Wang XF, Ma ZY, Zhang GY, Han GY. Somatic embryogenesis and plant regeneration from two recalcitrant genotypes of Gossypium hirsutum L. Agri Sci China. 2006;5(5):323-329.
Zobel A, Kura M, Tykarska T. Cytoplasmic and apoplastic location of phenolic compounds in the covering tissue of the Brassica napus radicle between embryogenesis and germination. Ann Bot. 1989;64:149-157.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Adriana M. Gallego, Luisa F. Rojas, Héctor A. Rodriguez, Conrado Mora, Lucía Atehortúa, Aura I. Urrea, Mark J. Guiltinan, Siela N. Maximova, Emmanuel Gaquerel, Martha Zuluaga, Natalia Pabón-Mora. (2019). Metabolomic profile of cacao cell suspensions growing in blue light/dark conditions with potential in food biotechnology. Plant Cell, Tissue and Organ Culture (PCTOC), 139(2), p.275. https://doi.org/10.1007/s11240-019-01679-3.
2. Alfaro-Corres Arnoldo Enrique, Lecona-Guzman Carlos Alberto, Guti閞rez-Miceli Federico Antonio, Mart韓ez-Esteves Manuel, Silverio-G髆ez Mar韆 del Carmen, Medina-Lara Mar韆 de F醫ima, Ruiz-Lau Nancy. (2023). Effect of Water Stress Induced by Polyethylene Glycol on Growth, Proline Accumulation in Agave americana L.. Phyton, 92(2), p.629. https://doi.org/10.32604/phyton.2022.023970.
3. Ana María Henao Ramírez, David Hernando Palacio Hajduk, Diana Maria Cano Martínez, Aura Inés Urrea Trujillo. (2023). Indicator framework for large-scale cacao (Theobroma cacao L.) in vitro plant production planning and controlling. Bionatura, 8(1), p.1. https://doi.org/10.21931/RB/2023.08.01.8.
4. Mar Asunción Garate-Navarro, Enrique Arévalo-Gardini. (2017). Induction of Somatic Embryogenesis from Cocoa Farmer Field Collection of ICT - Peru. International Annals of Science, 2(1), p.6. https://doi.org/10.21467/ias.2.1.6-11.
5. Ana María Henao Ramírez, David Hernando Palacio Hajduk, Aura Inés Urrea Trujillo. (2022). Cost Analysis of Cacao (Theobroma cacao L.) Plant Propagation through the Somatic Embryogenesis Method. Bionatura, 7(2), p.1. https://doi.org/10.21931/RB/2022.07.02.2.
6. Ana Maria Henao Ramirez, Diana Maria Cano Martinez, Rodrigo Alberto Hoyos Sánchez, Aura Inés Urrea Trujillo. (2023). Propagation of cacao ( Theobroma cacao L.) clone ‘CCN51’ using somatic embryogenesis: from pilot scale to commercial production . The Journal of Horticultural Science and Biotechnology, 98(6), p.814. https://doi.org/10.1080/14620316.2023.2205855.
7. Tatiana Osorio Montoya, Ana María Henao Ramírez, Tatiana de la Hoz Vasquez, Aura Inés Urrea Trujillo. (2022). Propagation of IMC67 Plants, Universal Cacao ( Theobroma Cacao L.) Rootstock via Somatic Embryogenesis . International Journal of Fruit Science, 22(1), p.78. https://doi.org/10.1080/15538362.2021.2023067.
8. Lillien Fajardo Rosabal, Yans Guardia Puebla, Suyén Rodríguez Pérez, Ursula M. Rosabal Cordoví, Juan J. Silva Pupo, Stefaan P.O. Werbrouck. (2022). Effects of salicylic acid on the production of polyphenols and the reducing power of Theobroma cacao calli. Current Research in Biotechnology, 4, p.47. https://doi.org/10.1016/j.crbiot.2021.12.005.
9. Gnanam Ramasamy, Sivachandran Ramasamy, Nagganatha Suganthan Ravi, Rajalakshmi Krishnan, Rajesh Subramanian, Renuka Raman, Sudhakar Duraialaguraja, Raveendran Muthurajan, Jegadeeswari Vellaichamy. (2022). Haploid embryogenesis and molecular detection of somatic embryogenesis receptor-like kinase (TcSERK) genes in sliced ovary cultures of cocoa (Theobroma cacao L.). Plant Biotechnology Reports, 16(3), p.283. https://doi.org/10.1007/s11816-022-00756-y.
10. Ana María Henao Ramírez, Tatiana de la Hoz Vasquez, Tatiana Marcela Ospina Osorio, Lucía Atehortúa Garcés, Aura Inés Urrea Trujillo. (2018). Evaluation of the potential of regeneration of different Colombian and commercial genotypes of cocoa ( Theobroma cacao L.) via somatic embryogenesis. Scientia Horticulturae, 229, p.148. https://doi.org/10.1016/j.scienta.2017.10.040.
11. Sulistyani Pancaningtyas, Rubiyo, C. Indrawanto. (2021). The evaluation of java fine flavor cocoa propagation through somatic embryogenesis technique for germplasm preservation. E3S Web of Conferences, 306, p.01056. https://doi.org/10.1051/e3sconf/202130601056.
12. Ana María Henao-Ramírez, Aura Inés Urrea-Trujillo. (2020). Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery. , p.63. https://doi.org/10.1007/978-3-030-51358-0_5.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).