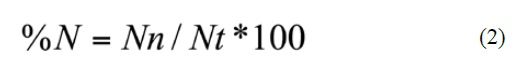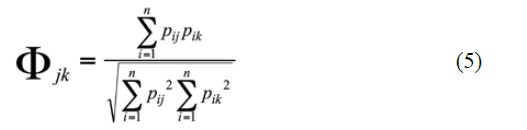DIET OF DIRECT-DEVELOPING FROGS (ANURA: CRAUGASTORIDAE: Pristimantis) FROM THE ANDES OF WESTERN COLOMBIA
DOI:
https://doi.org/10.15446/abc.v20n1.41153Palabras clave:
Colombia, diet, niche breadth, niche overlap, Pristimantis, Western Cordillera (es)
Descargas
We studied the diet of 15 montane frog species of the genus Pristimantis (Craugastoridae) from the Andes of Western Colombia to determine the diet range, breadth of niche and overlap among species. We identified 499 prey items from stomach and intestinal contents of 154 specimens. Prey items were included in 74 different categories. The most common 15 prey categories accounted for 65 % of all frogs’ diet. The invertebrate families Isotomidae, Chironomidae, Formicidae, and Tipulidae were the most abundant categories and accounted for 32 % of the frogs’ diet. Ten of the 15 frog species were found with at least one item of Araneae. Coleoptera and Tipulidae were found in nine frog species, and Acari and Carabidae in eight frog species. In general, beetles were found in gastrointestinal tracts of all species examined, except for P. quantus, but interpretation needs caution because only one individual of this species was caught. Pristimantis hectus showed a specialized diet, consuming mainly dipterans of the family Chironomidae, while the remaining species showed a generalist diet. Pristimantis palmeri showed niche overlap with P. erythropleura (Фjk = 0.69), P. myops (Фjk = 0.64), and P. orpacobates (Фjk = 0.64). Our results suggest that most of the frogs species studied are generalist, foraging opportunistically on dipterans, arachnids, collembolans, coleopterans, and hymenopterans. Here, we report the diet of montane Pristimantis species and discuss the results in comparison with data on related species in montane and lowland regions
Dieta de ranas de desarrollo directo (Anura: Craugastoridae: Pristimantis) de los Andes occidentales de Colombia
Estudiamos la dieta de 15 especies de ranas montanas del género Pristimantis (Craugastoridae) en la Cordillera Occidental de Colombia para determinar su variación, amplitud y traslape de nicho. En los contenidos estomacales e intestinales de 154 especímenes se identificaron 499 presas que fueron organizadas en 74 categorías diferentes. Las 15 categorías de presas más comunes representaron el 65 % de la dieta. Las familias de invertebrados Isotomidae, Chironomidae, Formicidae y Tipulidae fueron las más abundantes, representando el 32 % de la dieta de las ranas. Diez de las 15 especies de ranas presentaron al menos un ítem de Araneae. Coleoptera y Tipulidae fueron otras categorías frecuentemente encontradas en los tractos digestivos de las ranas (nueve especies de ranas presentaron estos ítems), seguidas de Acari y Carabidae (ocho especies). En general, los escarabajos fueron encontrados en los tractos gastrointestinales de todas las especies, excepto en P. quantus, pero este resultado debe tratarse con precaución porque solo un individuo de esta especie fue capturado. Pristimantis hectus mostró una dieta especializada consumiendo principalmente dípteros de la familia Chironomidae, mientras que las especies restantes mostraron una dieta generalista. Pristimantis palmeri presentó superposiciones de nicho con P. erythropleura (Фjk = 0,69), P. myops (Фjk = 0,64), y P. orpacobates (Фjk = 0,64). Nuestros resultados sugieren que la mayoría de las especies estudiadas son generalistas que se alimentan de manera oportunista de dípteros, arácnidos, colémbolos, coleópteros e himenópteros. Aquí, reportamos la dieta de especies montanas de Pristimantis y discutimos los resultados con datos de otros estudios en especies relacionadas de regiones montanas y tierras bajas
Artículo de investigación
DIET OF DIRECT-DEVELOPING FROGS (ANURA: CRAUGASTORIDAE: Pristimantis) FROM THE ANDES OF WESTERN COLOMBIA
Dieta de ranas de desarrollo directo (Anura: Craugastoridae: Pristimantis) de los Andes occidentales de Colombia
Juan Carlos GARCIA-R1, Carmen Elisa POSSO-GÓMEZ2, Heiber CÁRDENAS-HENAO2.
1Massey University, Institute of Agriculture and Environment, Massey University, Private Bag 11-222, Palmerston North, New Zealand.
2Departamento de Biología, Universidad del Valle, Cali, Colombia.
For correspondence. j.c.garciaramirez@massey.ac.nz
Received 11th December 2013, Returned 5th April for revisión 2014, Accepted 23rd May 2014.
Citation / Citar este artículo como: Garcia-R JC, Posso-Gómez CE, Cárdenas-Henao H. Diet of direct-developing frogs (Anura: Craugastoridae: Pristimantis) from the Andes of western Colombia. Acta biol. Colomb. 2015;20(1):79-87. doi: https://doi.org/10.15446/abc.v20n1.41153.
ABSTRACT
We studied the diet of 15 montane frog species of the genus Pristimantis (Craugastoridae) from the Andes of Western Colombia to determine the diet range, breadth of niche and overlap among species. We identified 499 prey items from stomach and intestinal contents of 154 specimens. Prey items were included in 74 different categories. The most common 15 prey categories accounted for 65 % of all frogs' diet. The invertebrate families Isotomidae, Chironomidae, Formicidae, and Tipulidae were the most abundant categories and accounted for 32 % of the frogs' diet. Ten of the 15 frog species were found with at least one item of Araneae. Coleoptera and Tipulidae were found in nine frog species, and Acari and Carabidae in eight frog species. In general, beetles were found in gastrointestinal tracts of all species examined, except for P. quantus, but interpretation needs caution because only one individual of this species was caught. Pristimantis hectus showed a specialized diet, consuming mainly dipterans of the family Chironomidae, while the remaining species showed a generalist diet. Pristimantis palmeri showed niche overlap with P. erythropleura (Фjk = 0.69), P. myops (Фjk = 0.64), and P. orpacobates (Фjk = 0.64). Our results suggest that most of the frogs species studied are generalist, foraging opportunistically on dipterans, arachnids, collembolans, coleopterans, and hymenopterans. Here, we report the diet of montane Pristimantis species and discuss the results in comparison with data on related species in montane and lowland regions.
Keywords: Colombia, diet, niche breadth, niche overlap, Pristimantis, Western Cordillera.
RESUMEN
Estudiamos la dieta de 15 especies de ranas montanas del género Pristimantis (Craugastoridae) en la Cordillera Occidental de Colombia para determinar su variación, amplitud y traslape de nicho. En los contenidos estomacales e intestinales de 154 especímenes se identificaron 499 presas que fueron organizadas en 74 categorías diferentes. Las 15 categorías de presas más comunes representaron el 65 % de la dieta. Las familias de invertebrados Isotomidae, Chironomidae, Formicidae y Tipulidae fueron las más abundantes, representando el 32 % de la dieta de las ranas. Diez de las 15 especies de ranas presentaron al menos un ítem de Araneae. Coleoptera y Tipulidae fueron otras categorías frecuentemente encontradas en los tractos digestivos de las ranas (nueve especies de ranas presentaron estos ítems), seguidas de Acari y Carabidae (ocho especies). En general, los escarabajos fueron encontrados en los tractos gastrointestinales de todas las especies, excepto en P. quantus, pero este resultado debe tratarse con precaución porque solo un individuo de esta especie fue capturado. Pristimantis hectus mostró una dieta especializada consumiendo principalmente dípteros de la familia Chironomidae, mientras que las especies restantes mostraron una dieta generalista. Pristimantis palmeri presentó superposiciones de nicho con P. erythropleura (Фjk = 0,69), P. myops (Фjk = 0,64), y P. orpacobates (Фjk = 0,64). Nuestros resultados sugieren que la mayoría de las especies estudiadas son generalistas que se alimentan de manera oportunista de dípteros, arácnidos, colémbolos, coleópteros e himenópteros. Aquí, reportamos la dieta de especies montanas de Pristimantis y discutimos los resultados con datos de otros estudios en especies relacionadas de regiones montanas y tierras bajas.
Palabras clave: amplitud de nicho, Colombia, Cordillera Occidental, dieta, Pristimantis, traslape de nicho.
INTRODUCTION
Understanding resource partitioning within and between species is important to determine the mechanism underlying the composition of ecological assemblages (Cody and Diamond, 1975). Ecological traits of coexisting species have provided explanations to the history and evolution of species diversity and the conformation and stability of communities (Cody and Diamond, 1975; Colston et al., 2010). Diet has been the main niche axis of interest to ecologists because competition for food can potentially shape community attributes such as number of coexisting species and their niche breadth and overlap (Diamond, 1975; Case, 1983; Toft, 1985).
Studies of prey type in anurans have been useful for determining the patterns of assemblage composition, ecological niches and organization of the species in their respective communities (Woolbright and Stewart, 1987; Ovaska, 1991; Alonso et al., 2001; Gutiérrez-L, 2003; Ortega et al., 2005). Moreover, anuran diets can help us to understand life history, patterns of behaviour, and the phylogenetic relationships between populations and clades (Wells, 1978; Lima and Magnusson, 1998; Sanabria et al., 2005; Juncá and Eterovick, 2007). This information is also important to predict the effects of decreasing populations in threatened worldwide anuran species and informing conservation plans for frog communities (Diamond, 1975; Young et al., 2001; Moritz, 2002; Vignoli and Luiselli, 2012).
Colombia hosts the highest species diversity of direct-developing frogs (Anura: Terrarana) in the world and the genus Pristimantis (family Craugastoridae) represents at least 27 % of the total anuran diversity in the country (AmphibiaWeb, 2014). A sizable number of Pristimantis is found in the Western Cordillera of the Andes, where it is the most common anuran group (Lynch, 1998a). The mountains of southwestern Colombia present unique anuran assemblages composed of more than 150 species above 1000 m a.s.l (Bernal and Lynch, 2008), but little is known about the diet of these species.
This study examined the diet composition of 15 montane frog's species of the genus Pristimantis in five localities of the Western Cordillera. Here, we used this data to analyse niche characteristics (food) and ecological correlations of niche preferences among species of local assemblages to answer the following questions: 1) What is the food composition of montane Pristimantis species in southwestern Colombia?, 2) Does diet composition vary among species?, and 3) How large are niche breadth and niche overlap of montane Pristimantis species in the Western Cordillera?
MATERIALS AND METHODS
Study area
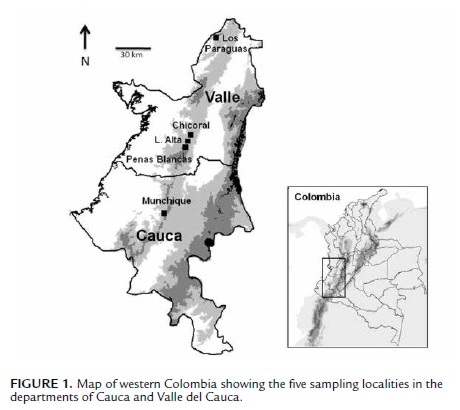
This study was conducted in Colombia at 1) Serranía de Los Paraguas (4#º50'N, 76#º15'W), municipality El Cairo, 2000– 2200 m a.s.l., between the Departments of Valle del Cauca and Chocó, 2) Loma Alta (3#º30'N, 76#º39'W), municipality Dagua, 1750–2100 m a.s.l., Department of Valle del Cauca, 3) Chicoral (3#º35'N, 76#º36'W), municipality La Cumbre, 1800–1900 m a.s.l., Department of Valle del Cauca, 4) Peñas Blancas, Farallones de Cali National Park (3#º25'N, 76#º39'), 1900–2200 m a.s.l., municipality Cali, Department of Valle del Cauca, and 5) La Romelia, Munchique National Park (2#º38', 76#º54'), 2400–2800 m a.s.l., municipality El Tambo, Department of Cauca (Fig. 1).
Sampling
Samples were collected on straight-line transects set up at each locality between 0.2 to 2.0 kilometres (km). Frog specimens were hand-collected using Visual Encounters Survey (Crump and Scott, 1994) from 19:00 h to 24:00 h. Euthanasia of frogs was performed with chloretone within a maximum of eight hours after capture. This study was carried out in strict accordance with evaluation of environmental, social, and educational impacts established at the Universidad del Valle (Convocatoria Interna number CI-746). Permit for sampling of specimens was obtained from Ministerio de Medio Ambiente, Vivienda y Desarrollo Territorial de Colombia (Resolución 573 del 10 de abril/08). Specimens were deposited at the herpetological collection – Universidad del Valle (Voucher numbers: UVC15812–915, UVC15917, UVC15919–24, UVC15927–52, UVC15955–71, UVC15975–79, UVC15982).
Gastrointestinal tracts (from stomach to cloaca) of each species were removed and fixed in 75 % ethanol. Prey items were sorted in a Petri dish, and later separated and categorized using a Nikon SMZ-645 stereoscope. Identification of prey items, depending on preservation of the prey, was held to the lowest possible taxonomic level using the dichotomous key by Triplehorn and Johnson (2005).
Diet composition
The quantitative importance of each prey group in the diet of Pristimantis was estimated using the index of relative importance (IRI) of Pinkas et al., (1971). The IRI provides a summary of dietary composition using the frequency of occurrence (% O) and percentage by number (% N) as follows:
Where n = number of gastrointestinal tracts that have the prey i, N = total number of analyzed gastrointestinal tracts, Nn = number of prey items of prey group n observed, and Nt = total number of prey items of all prey groups. The volume and length of the prey were not used because they were found disarticulated and highly digested.
Analysis of niche breadth was performed on species with n ≥ 5 individuals. Niche breadth of diet was calculated using Levins' index (1968) from the absolute frequencies (pi) of the number of prey categories (n):
We standardized the scale from 0 to 1 to compare the rates of niche breadth among species of Pristimantis, where 0 correspond to specialist species and 1 generalist species (Pereira et al., 2004). We used the formula of Levins' standardized index (Krebs, 1989) from the value calculated above (3) and the number of prey categories (n):
Niche overlap was performed on Pristimantis species with n ≥ 5 individuals focusing on species with sympatric distributions according to Lynch (1998b), and constructing a numerical abundance dietary matrix of prey items using Pianka index (Pianka, 1974):
Where Фjk = niche overlap between species j and k, Pij and Pik = proportions of predator j and k with prey i in their stomachs or digestive tracts. Diet overlap increases as the Pianka index increases and overlap is generally considered to be significantly when greater than 0.60 (Zaret and Rand, 1971; Calderón-Arguedas and Troyo, 2007).
RESULTS Diet composition
We examined 154 gastrointestinal tracts from 15 species of Pristimantis (Supplementary Table S1), of which only 13 gastrointestinal tracts were found empty. A total of 499 prey items were identified and placed into 74 categories (Table S1): two classes, 14 orders, four suborders, 53 families, and one category named "other arthropods" (OA) when identification was not possible. Occasionally, plant materials were found such as leaves, branches or seeds. Overall, for the species studied, between one (P. quantus and P. w-nigrum) and 38 (P. palmeri) prey categories were found. Sample sizes of P. calcaratus, P. achatinus, P. juanchoi, and P. w-nigrum were small (1 – 3 individuals) and a few prey items were found (1 – 7 items; Table S1). Araneae was the most frequent category found in gastrointestinal tracts. Ten of the 15 frog species had at least one item of Araneae, followed by Coleoptera and Tipulidae (nine frog species), and Acari and Carabidae (eigth frog species).
The prey items with the highest frequency of occurrence (% O) were found within the families Isotomidae (35.1 %), Chironomidae (26 %), and Formicidae (20.8 %). These items were mainly found in Pristimantis sp., P. acatallelus, P. hectus and P. palmeri. However, Formicidae was the most abundant item in P. myops (25 %), P. erythropleura (15.6 %), and P. orpacobates (15.6 %). Dipterans were the most abundant arthropods (25.7 %) followed by arachnids (16.6 %), coleopterans (13.4 %), and hymenopterans (11 %). Species with high amount of dipterans in their diet were P. hectus (32 items), P. acatallelus (23 items), P. brevifrons (16 items), P. palmeri (16 items), P. jubatus (13 items) and P. angustilineatus (10 items). Pristimantis palmeri and P. jubatus contained the greatest amount of coleopterans (22 and eight items, respectively) and hymenopterans (15 and 11 items, respectively), while P. jubatus and P. acatallelus contained most of the prey items in Araneae (10 and nine items, respectively). Beetles were observed in gastrointestinal tracts for all species with the exception of P. quantus, but only one specimen was collected for this species.
Intermediate and high values of niche breadth standardized index were found for most of the Pristimantis species evaluated, indicating a generalist diet (Table 1). However, P. hectus showed a specialized diet, consuming mainly dipterans of the family Chironomidae; almost half of the items (28 of 57 items) found in P. hectus were identified as belonging to this family. Furthermore, eight species showed exclusive consumption for some prey categories (Table S1). For example, Anapidae, Silvanidae, Neelidae, Forficullidae, Dipsocoridae, and Diplopoda were only consumed by P. acatallelus; Scarabeidae, Anobiidae, and Otitidae were only found in P. brevifrons; Tenebrionidae, Elateridae, Membracidae, Chalcididae, and Apocrita were exclusively found within P. jubatus individuals, while Bostrichidae, Miridae, Meinertellidae, Opiliones, and Homoptera were found in P. palmeri.
Three niche overlaps were discovered among species evaluated (Table 2): P. palmeri vs P. erythropleura (Фjk = 0.69), P. palmeri vs P. myops (Фjk = 0.64), and P. palmeri vs P. orpacobates (Фjk = 0.64). Pristimantis palmeri shared with P. erythropleura, P. myops and P. orpacobates a large amount of food items in the categories Formicidae, Acari, Araneae, Coleoptera, and Tipulidae.
DISCUSSION
The diet of Pristimantis is composed of a variety of arthropods but mostly dipterans (128 total items), arachnids (83 total items), collembolans (70 total items), coleopterans (67 total items), and hymenopterans (55 total items) which are abundant in leaf litter (especially collembolans, spiders and beetles of the family Carabidae) and low vegetation (Johnson and Catley, 2005). While most species in our study were generalists, P. hectus has a specialist diet consuming mainly dipterans, which are an abundant food resource in tropical rainforests (Didham, 1997). Pristimantis hectus has a diurnal foraging activity in low vegetation below 50 cm (Lynch and Duellman, 1997; García-R et al., 2005; García-R and Lynch, 2006). These characteristics, diurnal activity and foraging in low strata, may offer an opportunity for niche partitioning and prevent food competition with species that are nocturnal.
Dietary overlaps of P. palmeri among P. erythropleura, P. myops and P. orpacobates suggest that these species may compete for food. However, this competitive pressure might be reduced if there are differences in prey size in relation to body size within and between species, as it is common in other vertebrates (Ashmole, 1968; Dickman, 1988; Weise et al., 2010), and if each frog species use different microhabitat strata. For instance, P. myops is a minute frog with a snout-vent length in males of 10 – 13 mm and females of 14 – 17 mm (Lynch, 1998b), while males of P. palmeri are approximately 20 mm and females 28 mm (Bolívar-G et al., 2011). A difference of twice the size may suggest that P. palmeri can eat larger prey items than P. myops and thereby avoid direct competition. In addition, ants are the main prey among these species, a source readily available, since they live in colonies and are slow and therefore easy to catch. Likewise, ants have a low nutrient content (Parmelee, 1999) and their chitinous body make them difficult to digest (Toft, 1985), which is probably the reason of large quantities consumed.
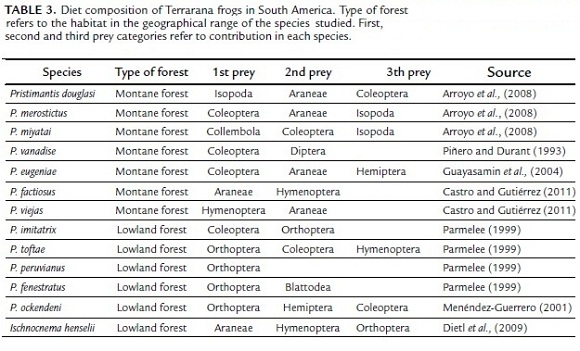
Terrarana frogs in South America (Table 3) have shown a wide spectrum of prey types with predominance of coleopterans and hymenopterans in their diets, including previous studies of Pristimantis in the Eastern Cordillera from the Andes of Colombia (Piñero and Durant, 1993; Parmelee, 1999; Menendez-Guerrero, 2001; Guayasamin et al., 2004; Arroyo et al., 2008; Dietl et al., 2009; Castro and Gutiérrez, 2011). Montane frogs are mainly insectivore generalists that choose their prey depending on microhabitat preferences and food availability (Ortega et al., 2005; Arroyo et al., 2008). However, the presence and abundance of prey items may vary by elevation and dominant vegetation type at each site. For example, according to Parmelee (1999), ants are an important component of the diet of several species of Pristimantis in lowland Amazonia. In contrast, ants provided a small contribution to the diet of the montane Pristimantis species studied here and did not contribute much to the diet of other species studied at high elevations (Table 3). The small contribution of ants in frog's diet at high altitudes is better explained by low ant density in montane forests compared to sub-montane and lowlands forests. Lower temperatures may affect ants' larvae development and food acquisition because of the low availability of food resources (Basset, 2001). Alternatively, it is also possible that the trophic opportunism for ants is more gradated within ecologically complex Pristimantis assemblages in relatively highland habitats.
CONCLUSION
Species of Pristimantis distributed in the montane regions of southwestern Colombia present a wide range of items consumed without strong differences in diet composition. The similarity of diets, however, did not indicate a marked competition because the high availability of food resources, suggesting an important factor for coexistence.
ACKNOWLEDGMENTS
We would like to thank the Ministerio de Medio Ambiente, Vivienda y Desarrollo Territorial de Colombia for research permission (Resolución 573 del 10 de abril/08) and the Universidad del Valle for funding (Convocatoria Interna CI746). Leonardo Lucas for help in the field and laboratory work. The rangers and inhabitants of Munchique National Park, Peñas Blancas, El Cairo, Chicoral, and Loma Alta, and owners of farms for access to some of the sampling locations. The fieldwork in Los Paraguas was facilitated by Serraniagua. Natasha Peters, Isabel Castro and Mariana Bulgarella helped in the translation of this manuscript. Thanks to Paul Gutiérrez and two anonymous reviewers for comments to improve this manuscript.
REFERENCES
AmphibiaWeb. Information on amphibian biology and conservation. [web application]. Berkeley, California: AmphibiaWeb. Available at: http://amphibiaweb.org/ (Accessed: May 25, 2014).
Alonso R, Rodríguez-Gómez A, Estrada AR. Patrones de actividad acústica y trófica de machos cantores de Eleutherodactylus eileenae (Anura: Leptodactylidae). Rev Esp Herp. 2001;15:45-52.
Arroyo S, Serrano-C V, Ramírez-P P. Diet, microhabitat and time of activity in a Pristimantis (Anura, Strabomantidae) assemblage. Phyllomedusa. 2008;7:109-119. Doi: 10.11606/issn.2316-9079.v7i2p109-119.
Ashmole N. Body size, prey size, and ecological segregation in five sympatric tropical terns (Aves: Laridae). Syst Biol. 1968;17: 292-304. Doi: 10.1093/sysbio/17.3.292.
Basset Y. Invertebrates in the canopy of tropical rain forests: how much do we really know?. Plant Ecol. 2001;153 (12):87-107. Doi: 10.1023/A:1017581406101.
Bernal MH, Lynch JD. Review and analysis of altitudinal distribution of the Andean anurans in Colombia. Zootaxa. 2008;1826:1-25.
Bolívar-G W, Giraldo A, Mendez J. Amphibia, Anura, Strabomantidae, Pristimantis palmeri Boulenger, 1912: Distribution extension for the Central Cordillera, Colombia. Check List. 2011;7:9-10.
Calderón-Arguedas O, Troyo A. Evaluación del nicho ecológico de formas larvales de Aedes aegypti y Culex quinquefasciatus (Diptera: Culicidae) en una comunidad urbana de San José, Costa Rica. Parasit Latinoam. 2007;64(3-4):142-147. Doi: 10.4067/S0717-77122007000200007.
Case TJ. Niche overlap and the assembly of island lizard communities. Oikos. 1983;41:427-433. Doi: 10.2307/3544102
Castro I, Gutiérrez P. Analisis de dieta de dos species de ranas Pristimantis (Anura: Strabomantidae) en un bosque montano de Anori, Antioquia (Colombia). VII Congreso Latinoamericano de Herpetologia y V Congreso Brasileiro de Herpetologia. Curitiba, Brasil. 2011.
Cody M, Diamond J. Ecology and evolution of communities. Harvard University Press; 1975. p. 560.
Colston T, Costa G, Vitt L. Snake diets and the deep history hypothesis. Biol J Linn Soc. 2010;101(2):476-486. Doi: 10.1111/j.1095-8312.2010.01502.x.
Crump M L, Scott NJ. Visual encounter surveys. In: Heyer WR, Donnelly MA, Mcdiarmid RW, Hayek LC, Foster MS, editors. Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institution Press; 1994. p. 84-92.
Diamond J. Assembly of species communities. In: Cody M, Diamond J, editors. Ecology and evolution of communities. Harvard University Press; 1975. p. 342-444.
Dickman C. Body size, prey size, and community structure in insectivorous mammals. Ecology. 1988;69:569-580. Doi: 10.2307/1941006.
Didham RK. Dipteran tree-crown assemblages in a diverse southern temperate rain forest. In: Stork NE, Adis J, Didham RK, editors Canopy arthropods. Chapman and Hall; 1997. p. 320-343.
Dietl J, Engels W, Solé M. Diet and feeding behaviour of the leaf-litter frog Ischnocnema henselii (Anura: Brachycephalidae) in Araucaria rain forests on the Serra Geral of Rio Grande do Sul, Brazil. J Nat Hist. 2009;43:23-24. Doi: 10.1080/00222930902898709.
García-R JC, Castro F, Cárdenas H. Relación entre la distribución de anuros y variables del hábitat en el sector La Romelia del Parque Nacional Natural Munchique (Cauca, Colombia). Caldasia. 2005;27:299-310.
García-R JC, Lynch JD. A new species of frog (genus Eleutherodactylus) from a cloud forest in Western Colombia. Zootaxa. 2006;1171:39–45.
Guayasamin JM, Bonaccorso E, Menéndez PA, Bustamante MR. Morphological variation, diet, and vocalization of Eleutherodactylus eugeniae (Anura: Leptodactylidae) with notes on its reproduction and ecology. Herp Rev. 2004;35:17-23.
Gutiérrez-L D. Composición, dieta, microhábitat y horas de actividad de los anuros presentes en dos tipos de bosque dentro del santuario de Fauna y Flora Guanentá, Alto Río Fonce. BSc Dissertation, Universidad Industrial de Santander, Colombia; 2003.
Johnson E, Catley K. La vida en la hojarasca. American Museum of Natural History; 2005. p. 28.
Juncá FA, Eterovick PC. Feeding ecology of two sympatric species of Aromobatidae, Allobates marchesianus and Anomaloglossus stepheni, in Central Amazon. J Herp. 2007;41(2):301-308. Doi: https://doi.org/10.1670/0022-1511(2007)41[301:FEOTSS]2.0.CO;2.
Krebs CJ. Ecological methodology. Harper and Rows Publishers; 1989. p. 654.
Levins R. Evolution in changing environments. Princeton University Press; 1968. p. 132.
Lima AP, Magnusson WE. Partitioning seasonal time: interactions among size, foraging activity and diet in leaf-litter frogs. Oecologia. 1998;116:259-266. Doi: 10.1007/s004420050587.
Lynch JD. La riqueza de la fauna anfibia de los Andes colombianos. Innovación y Ciencia. 1998a;7:46-51.
Lynch JD. New species of Eleutherodactylus from the Cordillera Occidental of western Colombia with a synopsis of the distributions of species in western Colombia. Rev Acad Col. 1998b;22:117-148.
Lynch JD, Duellman WE. Frogs of the genus Eleutherodactylus in western Ecuador: systematics, ecology, and biogeography. U Kansas Nat Hist Mus, Special Publication. 1997;23:1-236.
Menéndez-Guerrero PA. Ecología trófica de la diversidad de anuros del Parque Nacional Yasuní en la Amazonía Ecuatoriana. BSc Dissertation, Pontificia Universidad Católica del Ecuador, Ecuador; 2001.
Moritz C. Strategies to protect biological diversity and the evolutionary processes that sustain it. Syst Biol. 2002;51:238-254. Doi: 10.1080/10635150252899752.
Ortega JE, Serrano VH, Ramírez-Pinilla MP. Diet composition and microhabitat of Eleutherodactylus johnstonei in an introduced population at Bucaramanga City, Colombia. Herp Rev. 2005;36:238–241.
Ovaska K. Diet of the frog Eleutherodactylus johnstonei (Leptodactylidae) in Barbados, West Indies. J Herp. 1991;25:486-488. Doi: 10.2307/1564775.
Parmelee JR. Trophic ecology of a tropical anuran assemblage. Sci Pap Nat Hist Mus U Kansas. 1999;11:1-59.
Pereira CC, Smith WS, Espíndola ELG. Hábitos alimenticios de nueve especies de peces del embalse de Três Irmãos, São Paulo, Brasil. Universidad y Ciencia. 2004;1:33-38.
Pianka ER. Niche overlap and diffuse competition. PNAS. 1974;71:2141-2145. Doi: 10.1073/pnas.71.5.2141.
Pinkas L, Oliphant MS, Iverson LR. Food habits of albacore, bluefin tuna, and bonito in California waters. Fish Bull. 1971;152: 1-105.
Piñero J, Durant P. Dieta y hábitat de una comunidad de anuros de selva nublada en los Andes merideños. Ecotrópicos. 1993;6:1-9.
Sanabria EA, Quiroga LB, Acosta JC. Dieta de Leptodactylus ocellatus (Linnaeus, 1758) (Anura: Leptodactylidae) en un humedal del oeste de Argentina. Rev Peru Biol. 2005;12:472-477.
Toft CA. Resource partitioning in amphibians and reptiles. Copeia. 1985;1985:1-21.
Triplehorn CA, Johnson NF. Borror and DeLong's introduction to the study of insects. Thomson Brooks/Cole; 2005. p. 864.
Vignoli L, Luiselli L. Dietary relationships among coexisting anuran amphibians: a worldwide quantitative review. Oecologia. 2012;169(2):499–509. Doi: 10.1007/s00442-011-2204-9.
Weise M, Harvey J, Costa D. The role of body size in individual-based foraging strategies of a top marine predator. Ecology. 2010;91(4):1004-1015. Doi: 10.1890/08-1554.1.
Wells KD. Territoriality in the green frog (Rana clamitans): vocalizations and agonistic behavior. Anim Behav. 1978;26:1051-1063. Doi: 10.1016/0003-3472(78)90094-5.
Woolbright LL, Stewart MM. Foraging success of the tropical frog Eleutherodactylus coqui: the cost of calling. Copeia. 1987;1987:69-75.
Young BE, Lips KR, Reaser JK, Ibáñez R, Salas AW, Cedeño JR, et al. Population Declines and Priorities for Amphibian Conservation in Latin America. Cons Biol. 2001.15(5):1213–1223. Doi: 10.1111/j.1523-1739.2001.00218.x.
Zaret TM, Rand SA. Competition in tropical stream fishes: support for the competitive exclusion principle. Ecology. 1971;52:336-342. Doi: 10.2307/1934593.
Referencias
AmphibiaWeb. Information on amphibian biology and conservation. [web application]. Berkeley, California: AmphibiaWeb. Available at: http://amphibiaweb.org/ (Accessed: May 25, 2014).
Alonso R, Rodríguez-Gómez A, Estrada AR. Patrones de actividad acústica y trófica de machos cantores de Eleutherodactylus eileenae (Anura: Leptodactylidae). Rev Esp Herp. 2001;15:45-52.
Arroyo S, Serrano-C V, Ramírez-P P. Diet, microhabitat and time of activity in a Pristimantis (Anura, Strabomantidae) assemblage. Phyllomedusa. 2008;7:109-119.
Ashmole N. Body size, prey size, and ecological segregation in five sympatric tropical terns (Aves: Laridae). Syst Biol. 1968;17: 292-304.
Basset Y. Invertebrates in the canopy of tropical rain forests: how much do we really know?. Plant Ecol. 2001;153:87-107.
Bernal MH, Lynch JD. Review and analysis of altitudinal distribution of the Andean anurans in Colombia. Zootaxa. 2008;1826:1-25.
Bolívar-G W, Giraldo A, Mendez J. Amphibia, Anura, Strabomantidae, Pristimantis palmeri Boulenger, 1912: Distribution extension for the Central Cordillera, Colombia. Check List. 2011;7:9-10.
Calderón-Arguedas O, Troyo A. Evaluación del nicho ecológico de formas larvales de Aedes aegypti y Culex quinquefasciatus (Diptera: Culicidae) en una comunidad urbana de San José, Costa Rica. Parasit Latinoam. 2007;62:142-147.
Case TJ. Niche overlap and the assembly of island lizard communities. Oikos. 1983;41:427-433.
Castro I, Gutiérrez P. Analisis de dieta de dos species de ranas Pristimantis (Anura: Strabomantidae) en un bosque montano de Anori, Antioquia (Colombia). VII Congreso Latinoamericano de Herpetologia y V Congreso Brasileiro de Herpetologia; 2011. Curitiba, Brasil.
Cody M, Diamond J. Ecology and evolution of communities. Harvard University Press; 1975. p. 560.
Colston T, Costa G, Vitt L. Snake diets and the deep history hypothesis. Biol J Linn Soc. 2010;101:476-486.
Crump M L, Scott NJ. Visual encounter surveys. In: Heyer WR, Donnelly MA, Mcdiarmid RW, Hayek LC, Foster MS, editors.
Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institution Press; 1994. p. 84-92.
Diamond J. Assembly of species communities. In: Cody M, Diamond J, editors. Ecology and evolution of communities. Harvard University Press; 1975. p. 342-444.
Dickman C. Body size, prey size, and community structure in insectivorous mammals. Ecology. 1988;69:569-580.
Didham RK. Dipteran tree-crown assemblages in a diverse southern temperate rain forest. In: Stork NE, Adis J, Didham RK, editors Canopy arthropods. Chapman and Hall; 1997. p. 320-343.
Dietl J, Engels W, Solé M. Diet and feeding behaviour of the leaf-litter frog Ischnocnema hen-selii (Anura: Brachycephalidae) in Araucaria rain forests on the Serra Geral of Rio Grande do Sul, Brazil. J Nat Hist. 2009;43:23-24.
García-R JC, Castro F, Cárdenas H. Relación entre la distribución de anuros y variables del hábitat en el sector La Romelia del Parque Nacional Natural Munchique (Cauca, Colombia). Caldasia. 2005;27:299-310.
García-R JC, Lynch JD. A new species of frog (genus Eleutherodactylus) from a cloud forest in Western Colombia. Zootaxa. 2006;1171:39–45.
Guayasamin JM, Bonaccorso E, Menéndez PA, Bustamante MR. Morphological variation, diet, and vocalization of Eleutherodactylus eugeniae (Anura: Leptodactylidae) with notes on its reproduction and ecology. Herp Rev. 2004;35:17-23.
Gutiérrez-L D. Composición, dieta, microhábitat y horas de actividad de los anuros presentes en dos tipos de bosque dentro del santuario de Fauna y Flora Guanentá, Alto Río Fonce. BSc Dissertation, Universidad Industrial de Santander, Colombia; 2003.
Johnson E, Catley K. La vida en la hojarasca. American Museum of Natural History; 2005. p. 28.
Juncá FA, Eterovick PC. Feeding ecology of two sympatric species of Aromobatidae, Allo-bates marchesianus and Anomaloglossus stepheni, in Central Amazon. J Herp. 2007;41:301-308.
Krebs CJ. Ecological methodology. Harper and Rows Publishers; 1989. p. 654.
Levins R. Evolution in changing environments. Princeton University Press; 1968. p. 132.
Lima AP, Magnusson WE. Partitioning seasonal time: interactions among size, foraging activi-ty and diet in leaf-litter frogs. Oecologia. 1998;116:259-266.
Lynch JD. La riqueza de la fauna anfibia de los Andes colombianos. Innovación y Ciencia. 1998a;7:46-51.
Lynch JD. New species of Eleutherodactylus from the Cordillera Occidental of western Colombia with a synopsis of the distributions of species in western Colombia. Rev Acad Col. 1998b;22:117-148.
Lynch JD, Duellman WE. Frogs of the genus Eleutherodactylus in western Ecuador: system-atics, ecology, and biogeography. U Kansas Nat Hist Mus, Special Publication. 1997;23:1-236.
Menéndez-Guerrero PA. Ecología trófica de la diversidad de anuros del Parque Nacional Yasuní en la Amazonía Ecuatoriana. BSc Dissertation, Pontificia Universidad Católica del Ecuador, Ecuador; 2001.
Moritz C. Strategies to protect biological diversity and the evolutionary processes that sustain it. Syst Biol. 2002;51:238-254.
Ortega JE, Serrano VH, Ramírez-Pinilla MP. Diet composition and microhabitat of Eleutherodactylus johnstonei in an introduced population at Bucaramanga City, Colombia. Herp Rev. 2005;36:238–241.
Ovaska K. Diet of the frog Eleutherodactylus johnstonei (Leptodactylidae) in Barbados, West Indies. J Herp. 1991;25:486-488.
Parmelee JR. Trophic ecology of a tropical anuran assemblage. Sci Pap Nat Hist Mus U Kansas. 1999;11:1-59.
Pereira CC, Smith WS, Espíndola ELG. Hábitos alimenticios de nueve especies de peces del embalse de Três Irmãos, São Paulo, Brasil. Universidad y Ciencia. 2004;1:33-38.
Pianka ER. Niche overlap and diffuse competition. PNAS. 1974;71:2141-2145.
Pinkas L, Oliphant MS, Iverson LR. Food habits of albacore, bluefin tuna, and bonito in California waters. Fish Bull. 1971;152: 1-105.
PIñero J, Durant P. Dieta y hábitat de una comunidad de anuros de selva nublada en los Andes merideños. Ecotrópicos. 1993;6:1-9.
Sanabria EA, Quiroga LB, Acosta JC. Dieta de Leptodactylus ocellatus (Linnaeus, 1758) (Anura: Leptodactylidae) en un humedal del oeste de Argentina. Rev Peru Biol. 2005;12:472-477.
Toft CA. Resource partitioning in amphibians and reptiles. Copeia. 1985;1985:1-21.
Triplehorn CA, Johnson NF. Borror and DeLong’s introduction to the study of insects. Thomson Brooks/Cole; 2005. p. 864.
Vignoli L, Luiselli L. Dietary relationships among coexisting anuran amphibians: a worldwide quantitative review. Oecologia. 2012;169:499–509.
Weise M, Harvey J, Costa D. The role of body size in individual-based foraging strategies of a top marine predator. Ecology. 2010;91:1004-1015.
Wells KD. Territoriality in the green frog (Rana clamitans): vocalizations and agonistic behavior. Anim Behav. 1978;26:1051-1063.
Woolbright LL, Stewart MM. Foraging success of the tropical frog Eleutherodactylus coqui: the cost of calling. Copeia. 1987;1987:69-75.
Young BE, Lips KR, Reaser JK, Ibáñez R, Salas AW, Cedeño JR, et al. Population declines and priorities for amphibian conservation in Latin America. Cons Biol. 2001;15:1213-1223.
Zaret TM, Rand SA. Competition in tropical stream fishes: support for the competitive exclu-sion principle. Ecology. 1971;52:336-342.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Thales Francisco S. S. Alves-dos-Santos, Lucas Rodriguez Forti, Marcelo Felgueiras Napoli. (2021). Feeding habits of the Robber Frog Pristimantis paulodutrai (Bokermann, 1975) in northeastern Brazil. Biota Neotropica, 21(2) https://doi.org/10.1590/1676-0611-bn-2020-1098.
2. Fillipe Pedroso-Santos, Igor Luis Kaefer, Patrick Ribeiro Sanches, Carlos Eduardo Costa-Campos. (2024). Trophic niche of four sympatric direct-developing frogs (Anura: Strabomantidae) from Eastern Brazilian Amazonia. Food Webs, 39, p.e00343. https://doi.org/10.1016/j.fooweb.2024.e00343.
3. Konrad Mebert, Macario González-Pinzón, Madian Miranda, Edgardo Griffith, Milan Vesely, P. Lennart Schmid, Abel Batista. (2022). A new rainfrog of the genus Pristimantis (Anura, Brachycephaloidea) from central and eastern Panama. ZooKeys, 1081, p.1. https://doi.org/10.3897/zookeys.1081.63009.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2015 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).